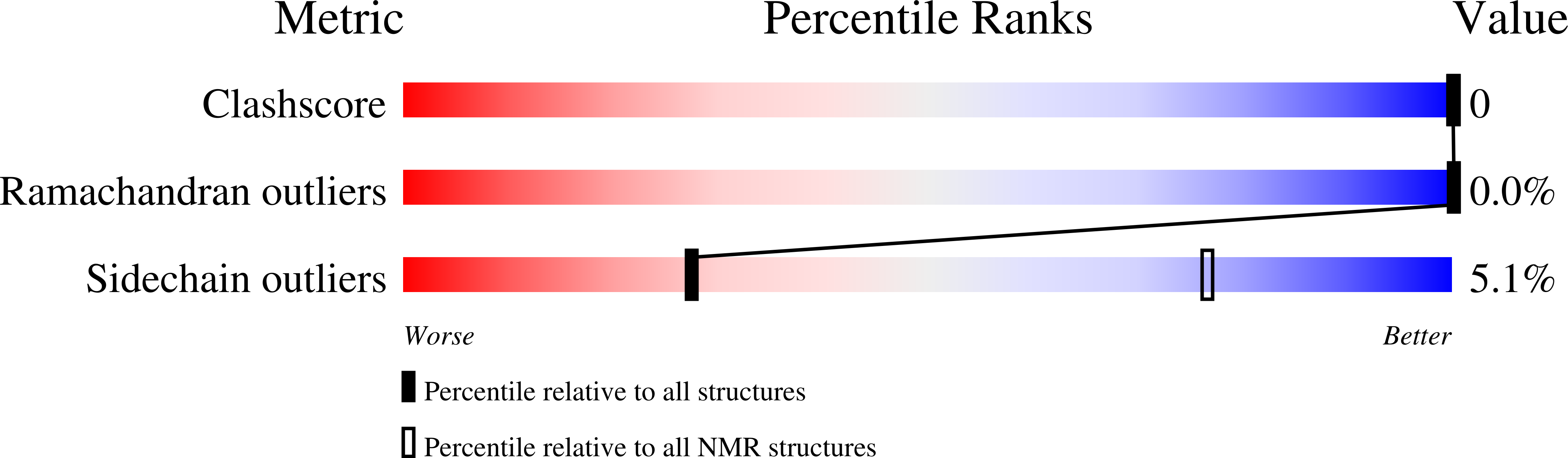Affinity switching of the LEDGF/p75 IBD interactome is governed by kinase-dependent phosphorylation.
Sharma, S., Cermakova, K., De Rijck, J., Demeulemeester, J., Fabry, M., El Ashkar, S., Van Belle, S., Lepsik, M., Tesina, P., Duchoslav, V., Novak, P., Hubalek, M., Srb, P., Christ, F., Rezacova, P., Hodges, H.C., Debyser, Z., Veverka, V.(2018) Proc Natl Acad Sci U S A 115: E7053-E7062
- PubMed: 29997176
- DOI: https://doi.org/10.1073/pnas.1803909115
- Primary Citation of Related Structures:
5YI9, 6EMO, 6EMP, 6EMQ, 6EMR - PubMed Abstract:
Lens epithelium-derived growth factor/p75 (LEDGF/p75, or PSIP1) is a transcriptional coactivator that tethers other proteins to gene bodies. The chromatin tethering function of LEDGF/p75 is hijacked by HIV integrase to ensure viral integration at sites of active transcription. LEDGF/p75 is also important for the development of mixed-lineage leukemia (MLL), where it tethers the MLL1 fusion complex at aberrant MLL targets, inducing malignant transformation. However, little is known about how the LEDGF/p75 protein interaction network is regulated. Here, we obtained solution structures of the complete interfaces between the LEDGF/p75 integrase binding domain (IBD) and its cellular binding partners and validated another binding partner, Mediator subunit 1 (MED1). We reveal that structurally conserved IBD-binding motifs (IBMs) on known LEDGF/p75 binding partners can be regulated by phosphorylation, permitting switching between low- and high-affinity states. Finally, we show that elimination of IBM phosphorylation sites on MLL1 disrupts the oncogenic potential of primary MLL1-rearranged leukemic cells. Our results demonstrate that kinase-dependent phosphorylation of MLL1 represents a previously unknown oncogenic dependency that may be harnessed in the treatment of MLL-rearranged leukemia.
Organizational Affiliation:
Molecular Virology and Gene Therapy, KU Leuven, 3000 Leuven, Belgium.