Crystal Structure of the Labile Complex of IL-24 with the Extracellular Domains of IL-22R1 and IL-20R2.
Lubkowski, J., Sonmez, C., Smirnov, S.V., Anishkin, A., Kotenko, S.V., Wlodawer, A.(2018) J Immunol 201: 2082-2093
- PubMed: 30111632
- DOI: https://doi.org/10.4049/jimmunol.1800726
- Primary Citation of Related Structures:
6DF3 - PubMed Abstract:
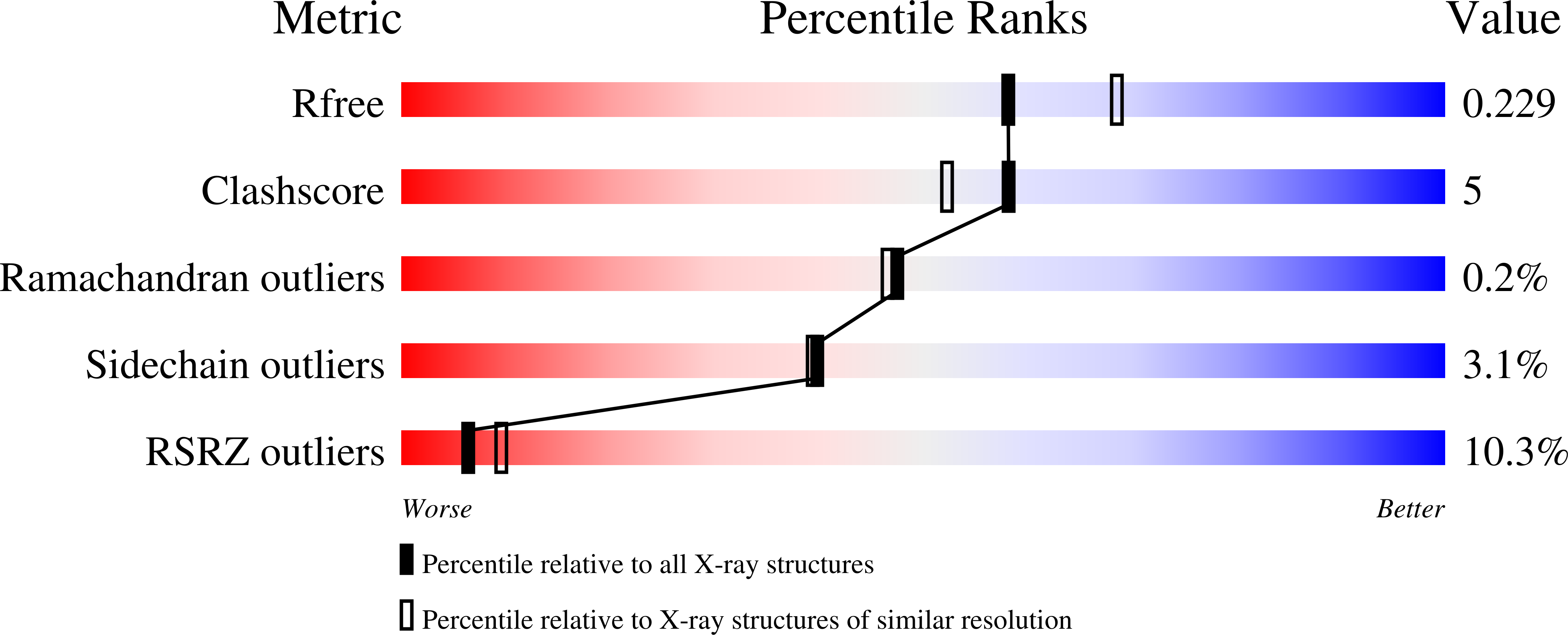
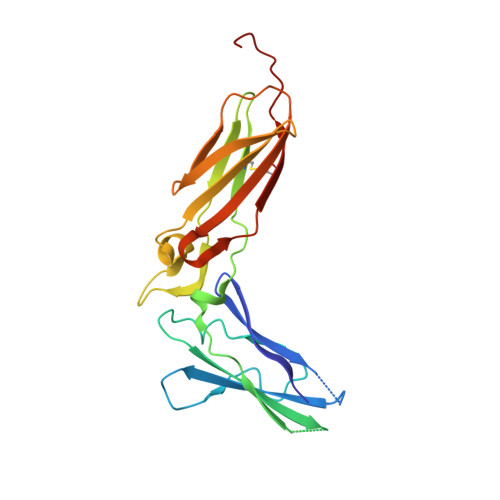
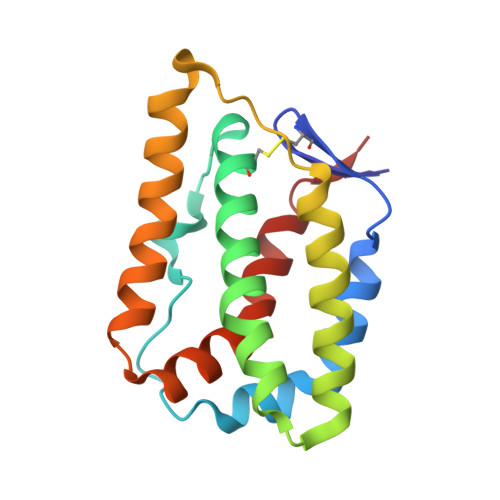
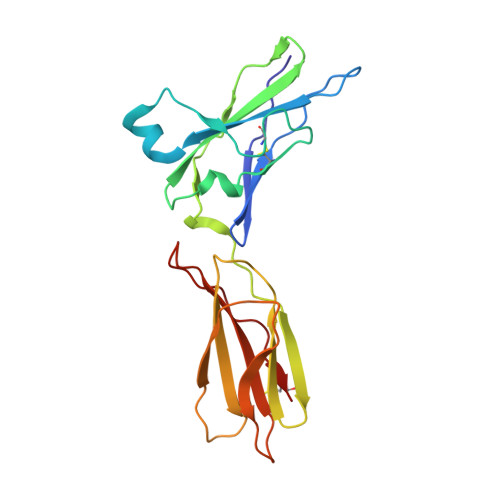
Crystal structure of the ternary complex of human IL-24 with two receptors, IL-22R1 and IL-20R2, has been determined at 2.15 Å resolution. A crystallizable complex was created by a novel approach involving fusing the ligand with a flexible linker to the presumed low-affinity receptor, and coexpression of this construct in Drosophila S2 cells together with the presumed high-affinity receptor. This approach, which may be generally applicable to other multiprotein complexes with low-affinity components, was necessitated by the instability of IL-24 expressed by itself in either bacteria or insect cells. Although IL-24 expressed in Escherichia coli was unstable and precipitated almost immediately upon its refolding and purification, a small fraction of IL-24 remaining in the folded state was shown to be active in a cell-based assay. In the crystal structure presented here, we found that two cysteine residues in IL-24 do not form a predicted disulfide bond. Lack of structural restraint by disulfides, present in other related cytokines, is most likely reason for the low stability of IL-24. Although the contact area between IL-24 and IL-22R1 is larger than between the cytokine and IL-20R2, calculations show the latter interaction to be slightly more stable, suggesting that the shared receptor (IL-20R2) might be the higher-affinity receptor.
Organizational Affiliation:
Macromolecular Crystallography Laboratory, Center for Cancer Research, National Cancer Institute, Frederick, MD 21702; lubkowsj@mail.nih.gov.