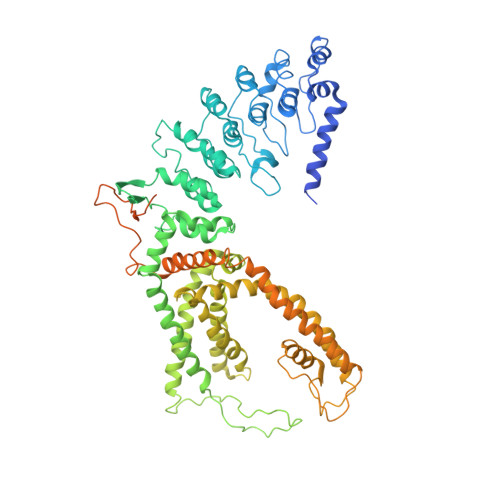
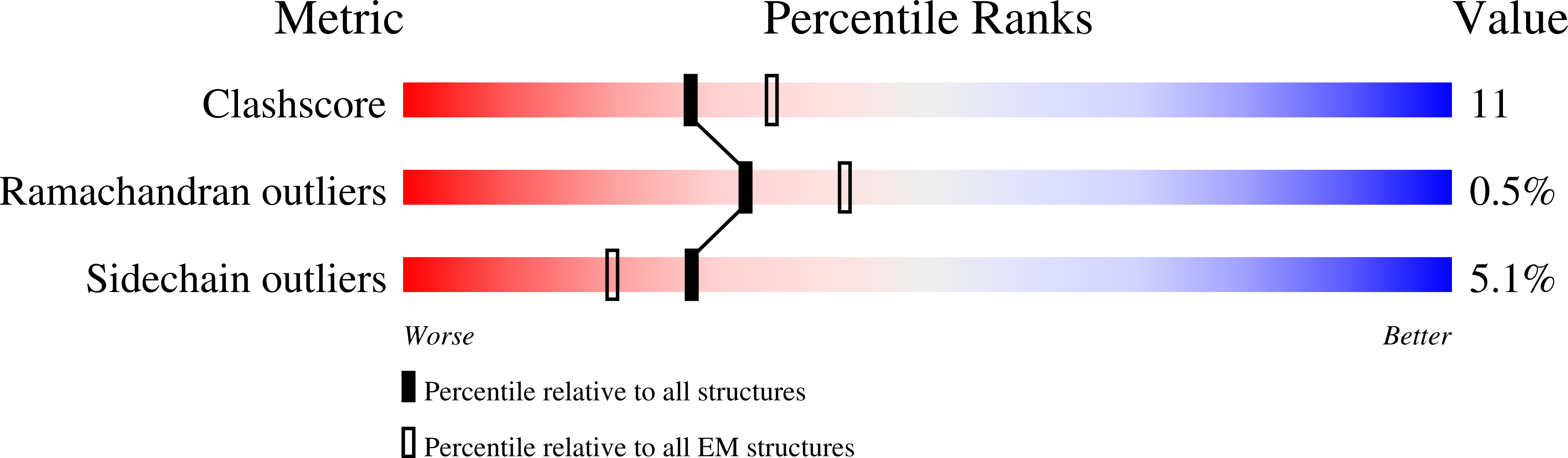Structural bases of TRP channel TRPV6 allosteric modulation by 2-APB.
Singh, A.K., Saotome, K., McGoldrick, L.L., Sobolevsky, A.I.(2018) Nat Commun 9: 2465-2465
- PubMed: 29941865
- DOI: https://doi.org/10.1038/s41467-018-04828-y
- Primary Citation of Related Structures:
6D7O, 6D7P, 6D7Q, 6D7S, 6D7T, 6D7V, 6D7X - PubMed Abstract:
Transient receptor potential (TRP) channels are involved in various physiological processes, including sensory transduction. The TRP channel TRPV6 mediates calcium uptake in epithelia and its expression is dramatically increased in numerous types of cancer. TRPV6 inhibitors suppress tumor growth, but the molecular mechanism of inhibition remains unknown. Here, we present crystal and cryo-EM structures of human and rat TRPV6 bound to 2-aminoethoxydiphenyl borate (2-APB), a TRPV6 inhibitor and modulator of numerous TRP channels. 2-APB binds to TRPV6 in a pocket formed by the cytoplasmic half of the S1-S4 transmembrane helix bundle. Comparing human wild-type and high-affinity mutant Y467A structures, we show that 2-APB induces TRPV6 channel closure by modulating protein-lipid interactions. Mutagenesis and functional analyses suggest that the identified 2-APB binding site might be present in other members of vanilloid subfamily TRP channels. Our findings reveal a mechanism of ion channel allosteric modulation that can be exploited for therapeutic design.
Organizational Affiliation:
Department of Biochemistry and Molecular Biophysics, Columbia University, 650 West 168th Street, New York, NY, 10032, USA.