Green fluorescent protein linked peptide inhibitor PMI in complex with mdm2
Chee, S.M.Q., Wongsantichon, J., Sana, B., Robinson, R.C., Ghadessy, F.J.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Green fluorescent protein linked MTide-02 | 358 | Aequorea victoria, Camelus dromedarius This entity is chimeric | Mutation(s): 0 Gene Names: GFP | 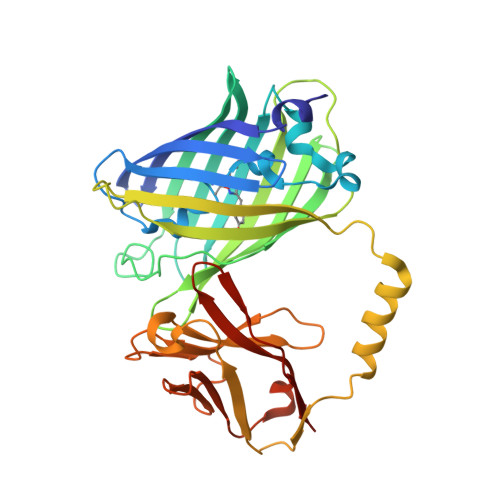 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| E3 ubiquitin-protein ligase Mdm2 | 122 | Homo sapiens | Mutation(s): 0 Gene Names: MDM2 EC: 2.3.2.27 | 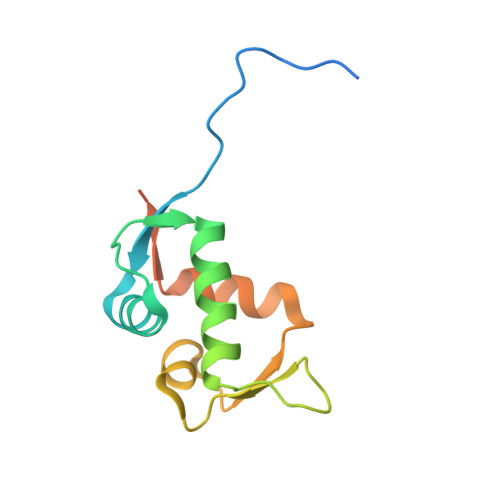 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q00987 (Homo sapiens) Explore Q00987 Go to UniProtKB: Q00987 | |||||
PHAROS: Q00987 GTEx: ENSG00000135679 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q00987 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PG4 Query on PG4 | E [auth B] | TETRAETHYLENE GLYCOL C8 H18 O5 UWHCKJMYHZGTIT-UHFFFAOYSA-N |  | ||
| PO4 Query on PO4 | D [auth A], F [auth B] | PHOSPHATE ION O4 P NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | ||
| GOL Query on GOL | C [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CRO Query on CRO | A | L-PEPTIDE LINKING | C15 H17 N3 O5 |  | THR, TYR, GLY |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 51.766 | α = 90 |
| b = 98.422 | β = 90 |
| c = 218.988 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHASER | phasing |