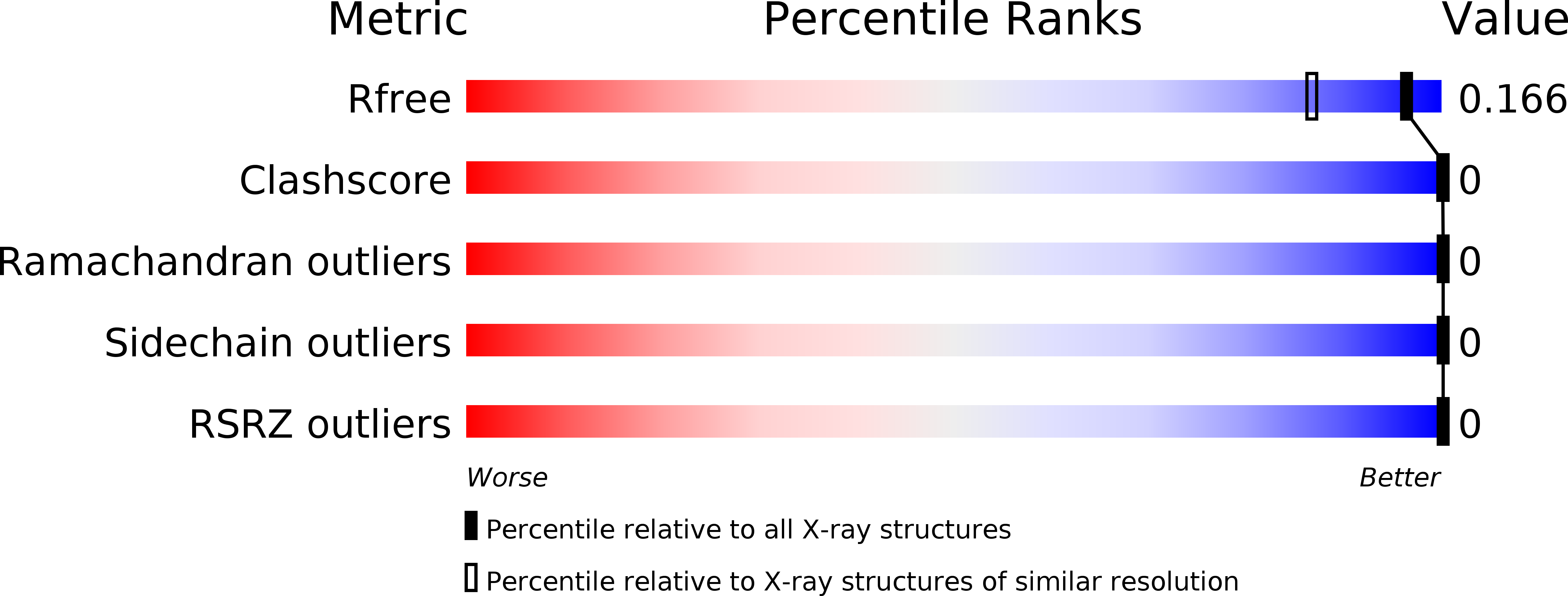
Atomic structures of corkscrew-forming segments of SOD1 reveal varied oligomer conformations.
Sangwan, S., Sawaya, M.R., Murray, K.A., Hughes, M.P., Eisenberg, D.S.(2018) Protein Sci 27: 1231-1242
- PubMed: 29453800
- DOI: https://doi.org/10.1002/pro.3391
- Primary Citation of Related Structures:
5WMJ, 5WOR, 6B79 - PubMed Abstract:
The aggregation cascade of disease-related amyloidogenic proteins, terminating in insoluble amyloid fibrils, involves intermediate oligomeric states. The structural and biochemical details of these oligomers have been largely unknown. Here we report crystal structures of variants of the cytotoxic oligomer-forming segment residues 28-38 of the ALS-linked protein, SOD1. The crystal structures reveal three different architectures: corkscrew oligomeric structure, nontwisting curved sheet structure and a steric zipper proto-filament structure. Our work highlights the polymorphism of the segment 28-38 of SOD1 and identifies the molecular features of amyloidogenic entities.
Organizational Affiliation:
Department of Biological Chemistry Los Angeles, Howard Hughes Medical Institute, UCLA-DOE and Molecular Biology Institute, California.