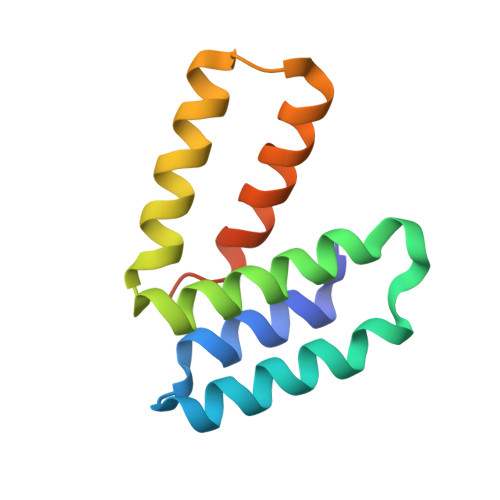
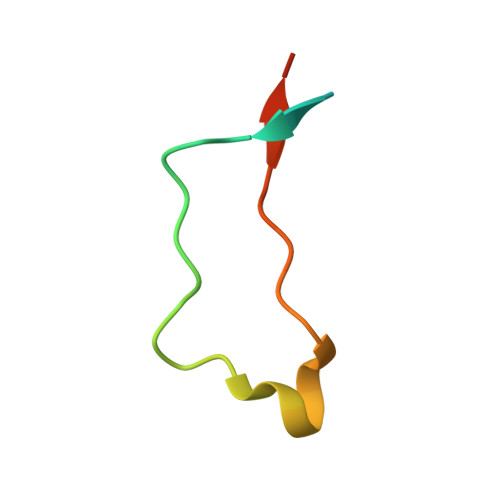
Mapping the ER Interactome: The P Domains of Calnexin and Calreticulin as Plurivalent Adapters for Foldases and Chaperones.
Kozlov, G., Munoz-Escobar, J., Castro, K., Gehring, K.(2017) Structure 25: 1415-1422.e3
- PubMed: 28877505
- DOI: https://doi.org/10.1016/j.str.2017.07.010
- Primary Citation of Related Structures:
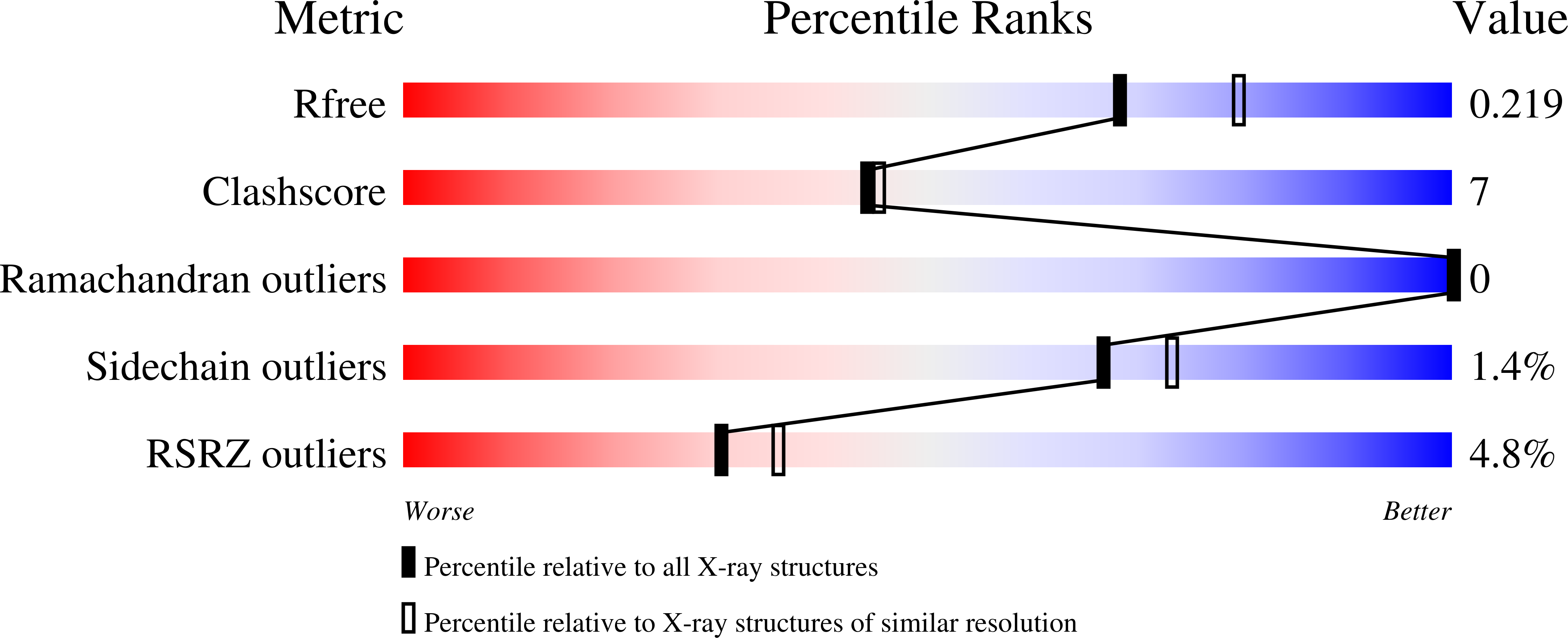
5V8Z, 5V90 - PubMed Abstract:
The lectin chaperones calreticulin (CRT) and calnexin (CNX) contribute to the folding of glycoproteins in the ER by recruiting foldases such as the protein disulfide isomerase ERp57 and the peptidyl prolyl cis-trans isomerase CypB. Recently, CRT was shown to interact with the chaperone ERp29. Here, we show that ERp29 directly binds to the P domain of CNX. Crystal structures of the D domain of ERp29 in complex with the P domains from CRT and calmegin, a tissue-specific CNX homolog, reveal a commonality in the mechanism of binding whereby the tip of the P domain functions as a plurivalent adapter to bind a variety of folding factors. We show that mutation of a single residue, D348 in CNX, abrogates binding to ERp29 as well as ERp57 and CypB. The structural diversity of the accessory factors suggests that these chaperones became specialized for glycoprotein folding through convergent evolution of their P-domain binding sites.
Organizational Affiliation:
Department of Biochemistry, Groupe de recherche axé sur la Structure des protéines, McGill University, 3649 Promenade Sir William Osler, Montréal, QC H3G 0B1, Canada.