Structure-Based Discovery of 4-(6-Methoxy-2-methyl-4-(quinolin-4-yl)-9H-pyrimido[4,5-b]indol-7-yl)-3,5-dimethylisoxazole (CD161) as a Potent and Orally Bioavailable BET Bromodomain Inhibitor.
Zhao, Y., Bai, L., Liu, L., McEachern, D., Stuckey, J.A., Meagher, J.L., Yang, C.Y., Ran, X., Zhou, B., Hu, Y., Li, X., Wen, B., Zhao, T., Li, S., Sun, D., Wang, S.(2017) J Med Chem 60: 3887-3901
- PubMed: 28463487
- DOI: https://doi.org/10.1021/acs.jmedchem.7b00193
- Primary Citation of Related Structures:
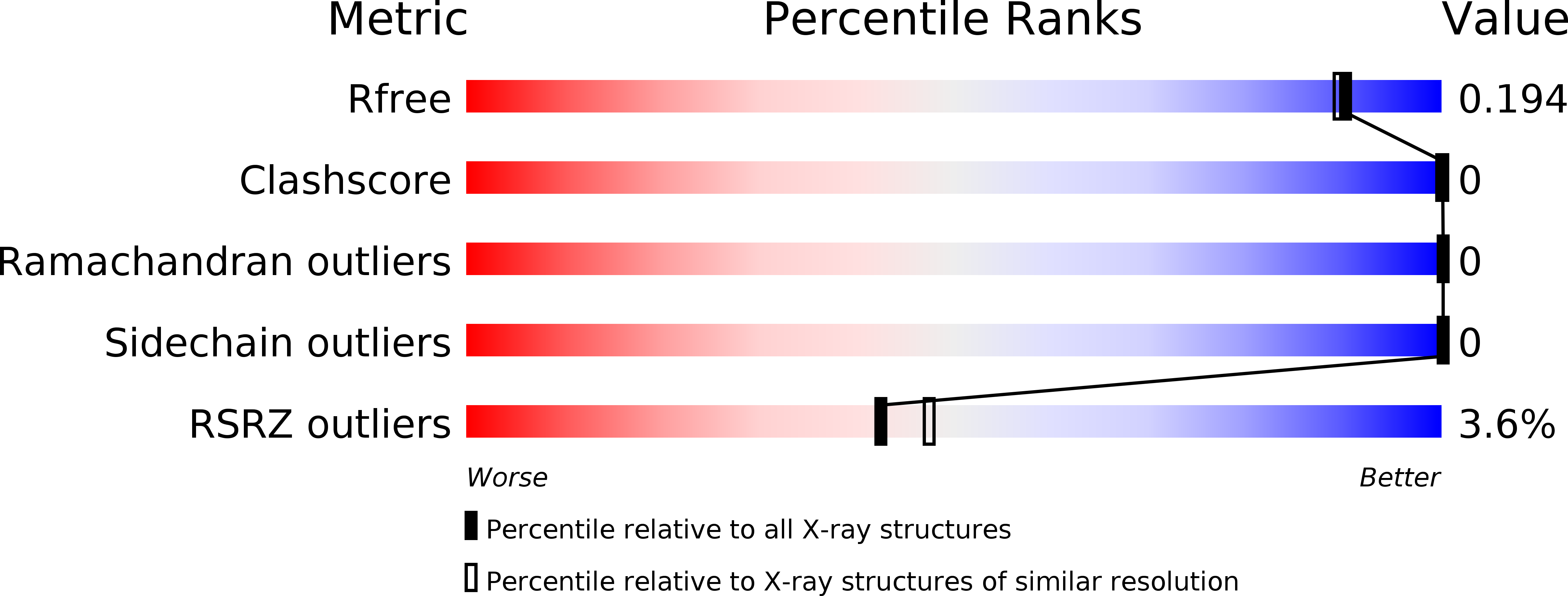
5UOO - PubMed Abstract:
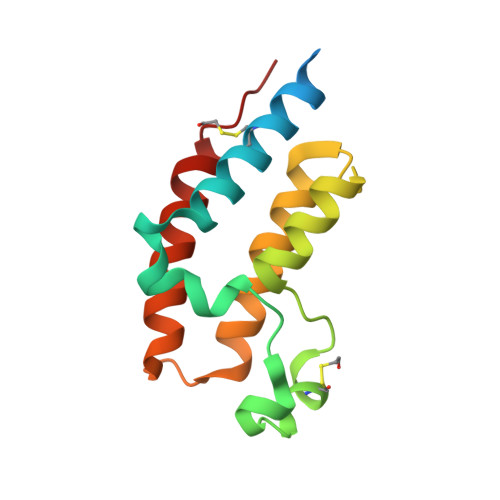
We have designed and synthesized 9H-pyrimido[4,5-b]indole-containing compounds to obtain potent and orally bioavailable BET inhibitors. By incorporation of an indole or a quinoline moiety to the 9H-pyrimido[4,5-b]indole core, we identified a series of small molecules showing high binding affinities to BET proteins and low nanomolar potencies in inhibition of cell growth in acute leukemia cell lines. One such compound, 4-(6-methoxy-2-methyl-4-(quinolin-4-yl)-9H-pyrimido[4,5-b]indol-7-yl)-3,5-dimethylisoxazole (31) has excellent microsomal stability and good oral pharmacokinetics in rats and mice. Orally administered, 31 achieves significant antitumor activity in the MV4;11 leukemia and MDA-MB-231 triple-negative breast cancer xenograft models in mice. Determination of the cocrystal structure of 31 with BRD4 BD2 provides a structural basis for its high binding affinity to BET proteins. Testing its binding affinities against other bromodomain-containing proteins shows that 31 is a highly selective inhibitor of BET proteins. Our data show that 31 is a potent, selective, and orally active BET inhibitor.
Organizational Affiliation:
Comprehensive Cancer Center and Departments of Internal Medicine, Pharmacology, and Medicinal Chemistry, University of Michigan , Ann Arbor, Michigan 48109, United States.