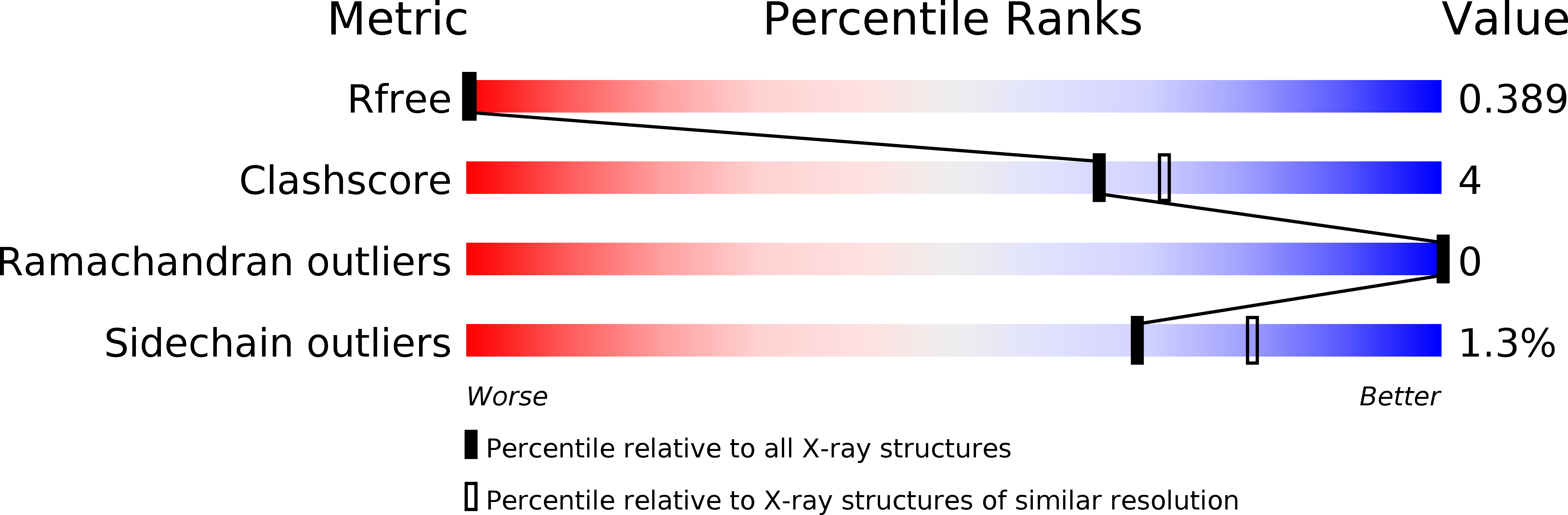PCNA dependent cellular activities tolerate dramatic perturbations in PCNA client interactions.
Wilson, R.H., Biasutto, A.J., Wang, L., Fischer, R., Baple, E.L., Crosby, A.H., Mancini, E.J., Green, C.M.(2017) DNA Repair (Amst) 50: 22-35
- PubMed: 28073635
- DOI: https://doi.org/10.1016/j.dnarep.2016.12.003
- Primary Citation of Related Structures:
5MOM - PubMed Abstract:
Proliferating cell nuclear antigen (PCNA) is an essential cofactor for DNA replication and repair, recruiting multiple proteins to their sites of action. We examined the effects of the PCNA S228I mutation that causes PCNA-associated DNA repair disorder (PARD). Cells from individuals affected by PARD are sensitive to the PCNA inhibitors T3 and T2AA, showing that the S228I mutation has consequences for undamaged cells. Analysis of the binding between PCNA and PCNA-interacting proteins (PIPs) shows that the S228I change dramatically impairs the majority of these interactions, including that of Cdt1, DNMT1, PolD3 p66 and PolD4 p12 . In contrast p21 largely retains the ability to bind PCNA S228I . This property is conferred by the p21 PIP box sequence itself, which is both necessary and sufficient for PCNA S228I binding. Ubiquitination of PCNA is unaffected by the S228I change, which indirectly alters the structure of the inter-domain connecting loop. Despite the dramatic in vitro effects of the PARD mutation on PIP-degron binding, there are only minor alterations to the stability of p21 and Cdt1 in cells from affected individuals. Overall our data suggests that reduced affinity of PCNA S228I for specific clients causes subtle cellular defects in undamaged cells which likely contribute to the etiology of PARD.
Organizational Affiliation:
Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Oxford, OX3 7BN, UK.