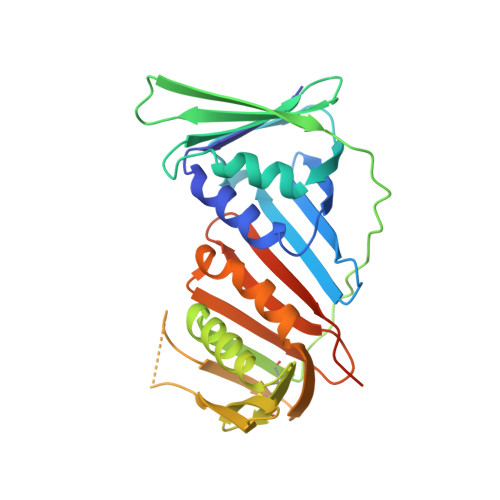
Crystal structure of human PCNA in complex with the PIP box of DVC1
Wang, Y., Xu, M., Jiang, T.(2016) Biochem Biophys Res Commun 474: 264-270
- PubMed: 27084448
- DOI: https://doi.org/10.1016/j.bbrc.2016.04.053
- Primary Citation of Related Structures:
5IY4 - PubMed Abstract:
In higher eukaryotes, DVC1 (SPRTN, Spartan or C1orf124) is implicated in the translesion synthesis (TLS) pathway. DVC1 localizes to sites of DNA damage, binds to the proliferating cell nuclear antigen (PCNA) via its conserved PCNA-interacting motif (PIP box), and associates with ubiquitin selective segregase p97 and other factors, thus regulating translesion synthesis polymerases. Here, we report the crystal structure of human PCNA in complex with a peptide ((321)SNSHQNVLSNYFPRVS(336)) derived from human DVC1 that contains a unique YF type PIP box. Structural analysis reveals the detailed PIP box-PCNA interaction. Interestingly, substitution of Y331 with Phe severely reduces its PCNA binding affinity. These findings offer new insights into the determinants of PIP box for PCNA binding.
Organizational Affiliation:
National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, 19A Yuquan Road, Shijingshan District, Beijing 100049, China.