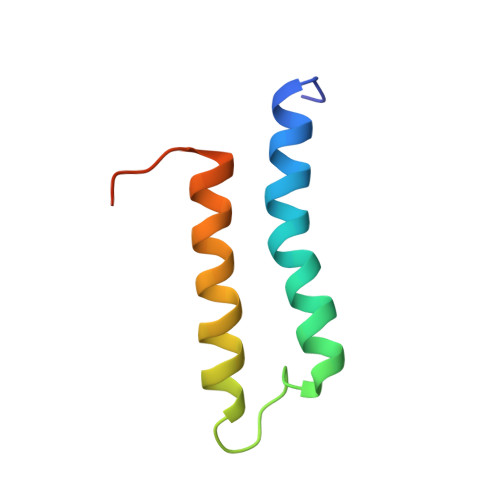
Solution structure of the microtubule-targeting COS domain of MID1.
Wright, K.M., Du, H., Dagnachew, M., Massiah, M.A.(2016) FEBS J 283: 3089-3102
- PubMed: 27367845
- DOI: https://doi.org/10.1111/febs.13795
- Primary Citation of Related Structures:
5IM8 - PubMed Abstract:
The human MID1 protein is required for the proper development during embryogenesis. Mutations of MID1 are associated with X-linked Opitz G syndrome, characterized by midline anomalies. MID1 associates with the microtubules and functions as an ubiquitin E3 ligase, targeting protein phosphatase 2A for ubiquitin-mediated regulation. The mechanism of microtubule association is not known. Recently, a 60-amino acid region termed the C-terminal subgroup One Signature (COS) box/domain was identified at the C-terminal end of the coiled-coil (CC) domain that facilitates microtubule localization. Insertion of the MID1 COS domain at the C-terminal end of the CC domain of a nonmicrotubule-associated TRIM protein confers microtubule localization. Here, we report the solution structure of the COS domain of MID1. The domain adopts a helix-loop-helix structure in which the N- and C-terminal ends are in close proximity. Hydrophobic residues stabilizing the interaction of the two α-helices form a central hydrophobic core. The loop separating the α-helices is structured, with two of its hydrophobic residues making contact with the central core. On the outer surface, positively charged residues form a distinct basic patch near the termini that we postulate is important for microtubule binding. A model of the structure of the preceding coiled-coil and COS domains (CC-COS) show that the COS domain forms a helical bundle at the C-terminal end of the CC domain similar to the spectrin-like fold observed with some known microtubule-binding proteins. Interestingly, the CC-COS domains bind to microtubules, demonstrating for the first time that MID1 can directly associate with the microtubules. Structural data are available in PDB database under the accession number 5IM8.
Organizational Affiliation:
Department of Chemistry and Center of Biomolecular Sciences, George Washington University, DC, USA.