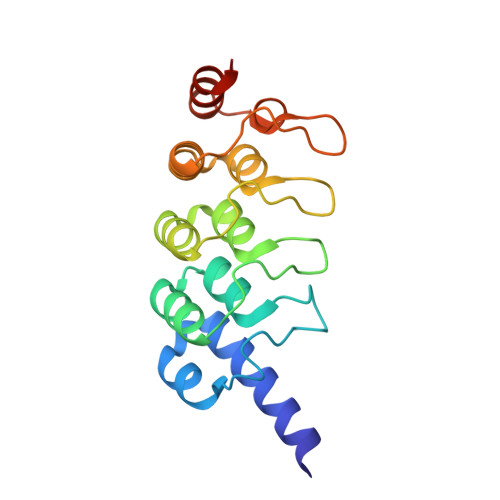
USP25 regulates Wnt signaling by controlling the stability of tankyrases
Xu, D., Liu, J., Fu, T., Shan, B., Qian, L., Pan, L., Yuan, J.(2017) Genes Dev 31: 1024-1035
- PubMed: 28619731
- DOI: https://doi.org/10.1101/gad.300889.117
- Primary Citation of Related Structures:
5GP7 - PubMed Abstract:
Aberrant activation of the Wnt signaling pathway plays an important role in human cancer development. Wnt signaling is negatively regulated by Axin, a scaffolding protein that controls a rate-limiting step in the destruction of β-catenin, the central activator of the Wnt pathway. In Wnt-stimulated cells, Axin is rapidly modified by tankyrase-mediated poly(ADP-ribosyl)ation, which promotes the proteolysis of Axin and consequent stabilization of β-catenin. Thus, regulation of the levels and activity of tankyrases is mechanistically important in controlling Wnt signaling. Here, we identify ubiquitin-specific protease 25 (USP25) as a positive regulator of Wnt/β-catenin signaling. We found that USP25 directly interacted with tankyrases to promote their deubiquitination and stabilization. We demonstrated that USP25 deficiency could promote the degradation of tankyrases and consequent stabilization of Axin to antagonize Wnt signaling. We further characterized the interaction between TNKS1 and USP25 by X-ray crystal structure determination. Our results provide important new insights into the molecular mechanism that regulates the turnover of tankyrases and the possibility of targeting the stability of tankyrases by antagonizing their interaction with USP25 to modulate the Wnt/β-catenin pathway.
Organizational Affiliation:
Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Pudong, Shanghai 201210, China.