Discovery of Novel Allosteric Non-Bisphosphonate Inhibitors of Farnesyl Pyrophosphate Synthase by Integrated Lead Finding.
Marzinzik, A.L., Amstutz, R., Bold, G., Bourgier, E., Cotesta, S., Glickman, J.F., Gotte, M., Henry, C., Lehmann, S., Hartwieg, J.C., Ofner, S., Pelle, X., Roddy, T.P., Rondeau, J.M., Stauffer, F., Stout, S.J., Widmer, A., Zimmermann, J., Zoller, T., Jahnke, W.(2015) ChemMedChem 10: 1884-1891
- PubMed: 26381451
- DOI: https://doi.org/10.1002/cmdc.201500338
- Primary Citation of Related Structures:
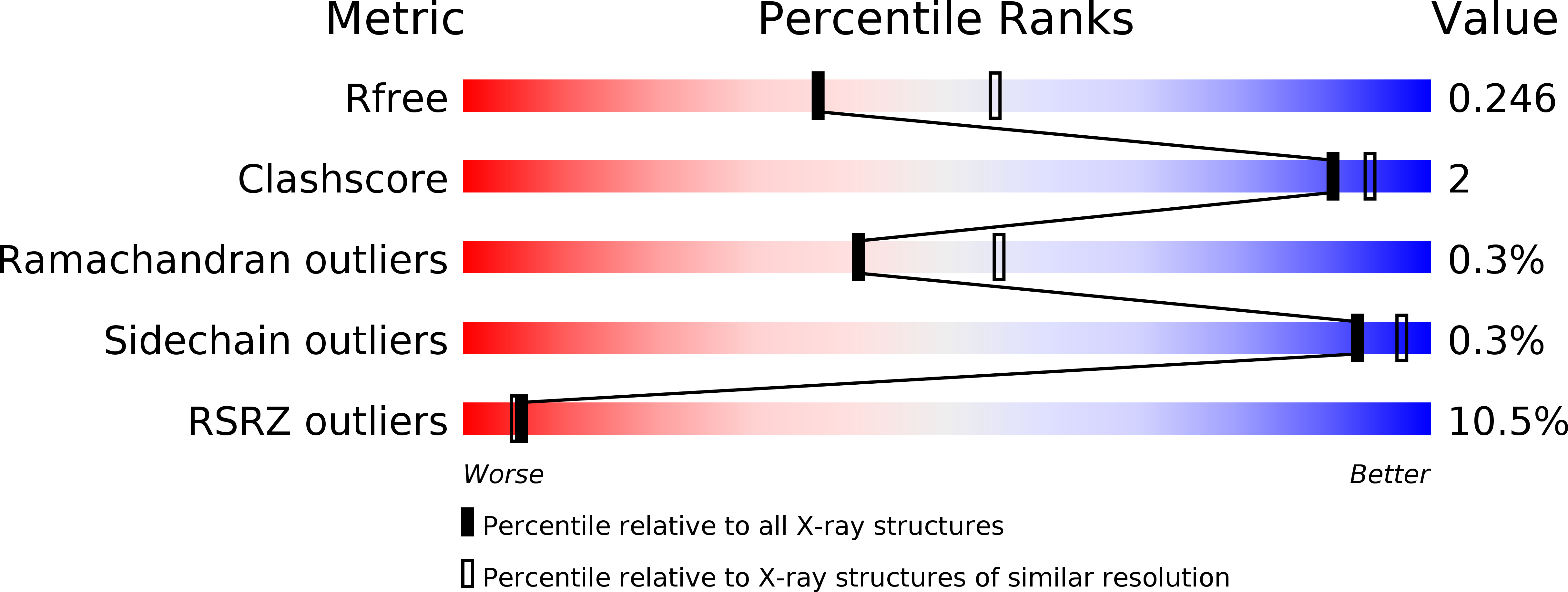
5DGN, 5DIQ, 5DJP, 5DJR, 5DJV - PubMed Abstract:
Farnesyl pyrophosphate synthase (FPPS) is an established target for the treatment of bone diseases, but also shows promise as an anticancer and anti-infective drug target. Currently available anti-FPPS drugs are active-site-directed bisphosphonate inhibitors, the peculiar pharmacological profile of which is inadequate for therapeutic indications beyond bone diseases. The recent discovery of an allosteric binding site has paved the way toward the development of novel non-bisphosphonate FPPS inhibitors with broader therapeutic potential, notably as immunomodulators in oncology. Herein we report the discovery, by an integrated lead finding approach, of two new chemical classes of allosteric FPPS inhibitors that belong to the salicylic acid and quinoline chemotypes. We present their synthesis, biochemical and cellular activities, structure-activity relationships, and provide X-ray structures of several representative FPPS complexes. These novel allosteric FPPS inhibitors are devoid of any affinity for bone mineral and could serve as leads to evaluate their potential in none-bone diseases.
Organizational Affiliation:
Novartis Institutes for BioMedical Research, Basel, 4002, Switzerland. wolfgang.jahnke@novartis.com.