Mapping Selective Inhibition of the Cancer-Related Carbonic Anhydrase IX Using Structure-Activity Relationships of Glucosyl-Based Sulfamates.
Mahon, B.P., Lomelino, C.L., Ladwig, J., Rankin, G.M., Driscoll, J.M., Salguero, A.L., Pinard, M.A., Vullo, D., Supuran, C.T., Poulsen, S.A., McKenna, R.(2015) J Med Chem 58: 6630-6638
- PubMed: 26203869
- DOI: https://doi.org/10.1021/acs.jmedchem.5b00845
- Primary Citation of Related Structures:
4ZWX, 4ZWY, 4ZWZ, 4ZX0, 4ZX1 - PubMed Abstract:
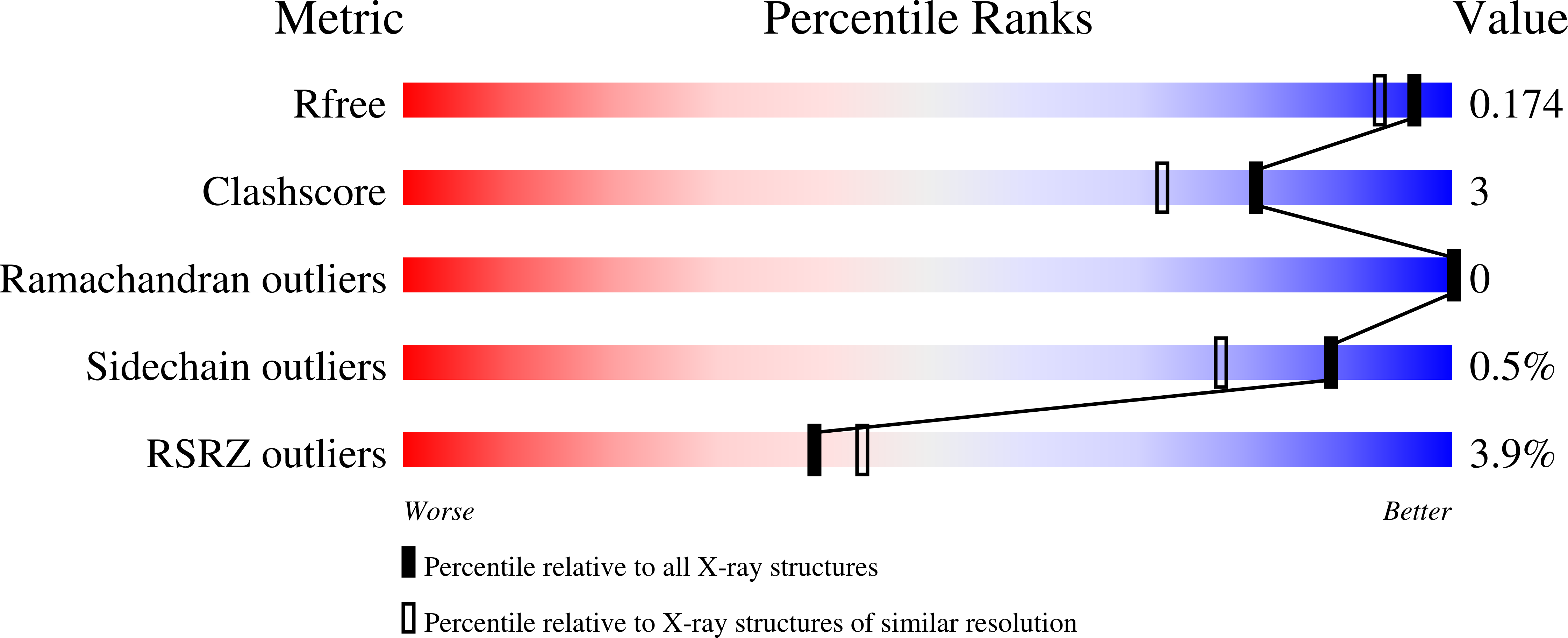
Inhibition of human carbonic anhydrase IX (hCA IX) has shown to be therapeutically advantageous for treating many types of highly aggressive cancers. However, designing selective inhibitors for hCA IX has been difficult due to its high structural homology and sequence similarity with off-target hCAs. Recently, the use of glucosyl sulfamate inhibitors has shown promise as selective inhibitors for hCA IX. In this study, we present five X-ray crystal structures, determined to a resolution of 1.7 Å or better, of both hCA II (a ubiquitous CA) and an engineered hCA IX-mimic in complex with selected glucosyl sulfamates and structurally rationalize mechanisms for hCA IX selectivity. Results from this study have allowed us, for the first time, to empirically "map" key interactions of the hCA IX active site in order to establish parameters needed to design novel hCA IX selective inhibitors.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, College of Medicine, University of Florida , 1600 SW Archer Road, PO Box 100245, Gainesville, Florida 32610, United States.