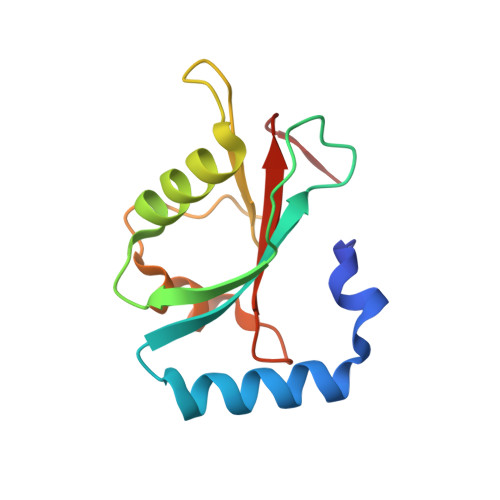
CUL3-KBTBD6/KBTBD7 Ubiquitin Ligase Cooperates with GABARAP Proteins to Spatially Restrict TIAM1-RAC1 Signaling.
Genau, H.M., Huber, J., Baschieri, F., Akutsu, M., Dotsch, V., Farhan, H., Rogov, V., Behrends, C.(2015) Mol Cell 57: 995-1010
- PubMed: 25684205
- DOI: https://doi.org/10.1016/j.molcel.2014.12.040
- Primary Citation of Related Structures:
4XC2 - PubMed Abstract:
The small Rho GTPase RAC1 is an essential regulator of cellular signaling that controls actin rearrangements and cell motility. Here, we identify a novel CUL3 RING ubiquitin ligase complex, containing the substrate adaptors KBTBD6 and KBTBD7, that mediates ubiquitylation and proteasomal degradation of TIAM1, a RAC1-specific GEF. Increasing the abundance of TIAM1 by depletion of KBTBD6 and/or KBTBD7 leads to elevated RAC1 activity, changes in actin morphology, loss of focal adhesions, reduced proliferation, and enhanced invasion. KBTBD6 and KBTBD7 employ ATG8 family-interacting motifs to bind preferentially to GABARAP proteins. Surprisingly, ubiquitylation and degradation of TIAM1 by CUL3(KBTBD6/KBTBD7) depends on its binding to GABARAP proteins. Our study reveals that recruitment of CUL3(KBTBD6/KBTBD7) to GABARAP-containing vesicles regulates the abundance of membrane-associated TIAM1 and subsequently spatially restricted RAC1 signaling. Besides their role in autophagy and trafficking, we uncovered a previously unknown function of GABARAP proteins as membrane-localized signaling scaffolds.
Organizational Affiliation:
Institute of Biochemistry II, Goethe University School of Medicine, 60590 Frankfurt am Main, Germany.