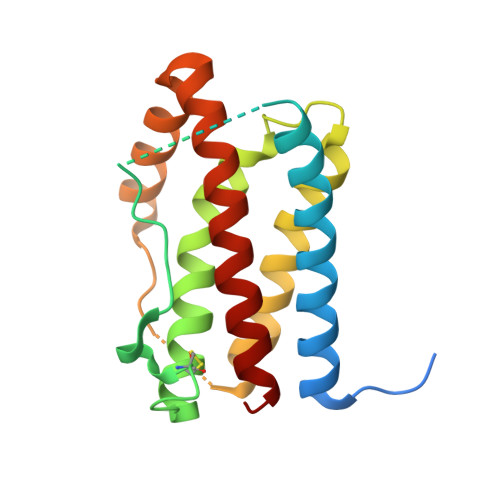
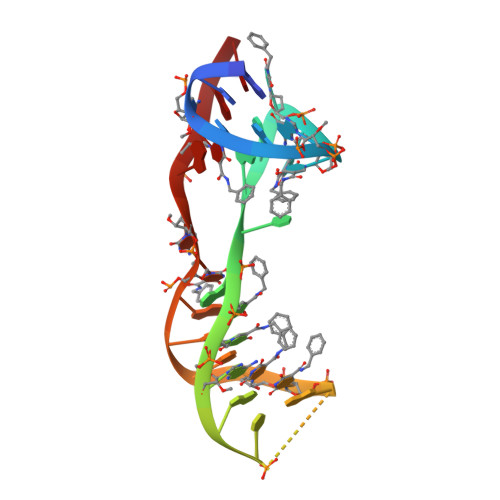
Crystal structure of interleukin-6 in complex with a modified nucleic Acid ligand.
Gelinas, A.D., Davies, D.R., Edwards, T.E., Rohloff, J.C., Carter, J.D., Zhang, C., Gupta, S., Ishikawa, Y., Hirota, M., Nakaishi, Y., Jarvis, T.C., Janjic, N.(2014) J Biol Chem 289: 8720-8734
- PubMed: 24415767
- DOI: https://doi.org/10.1074/jbc.M113.532697
- Primary Citation of Related Structures:
4NI7, 4NI9 - PubMed Abstract:
IL-6 is a secreted cytokine that functions through binding two cell surface receptors, IL-6Rα and gp130. Because of its involvement in the progression of several chronic inflammatory diseases, IL-6 is a target of pharmacologic interest. We have recently identified a novel class of ligands called SOMAmers (S low Off-rate Modified Aptamers) that bind IL-6 and inhibit its biologic activity. SOMAmers exploit the chemical diversity of protein-like side chains assembled on flexible nucleic acid scaffolds, resulting in an expanded repertoire of intra- and intermolecular interactions not achievable with conventional aptamers. Here, we report the co-crystal structure of a high affinity SOMAmer (Kd = 0.20 nm) modified at the 5-position of deoxyuridine in a complex with IL-6. The SOMAmer, comprised of a G-quartet domain and a stem-loop domain, engages IL-6 in a clamp-like manner over an extended surface exhibiting close shape complementarity with the protein. The interface is characterized by substantial hydrophobic interactions overlapping the binding surfaces of the IL-6Rα and gp130 receptors. The G-quartet domain retains considerable binding activity as a disconnected autonomous fragment (Kd = 270 nm). A single substitution from our diversely modified nucleotide library leads to a 37-fold enhancement in binding affinity of the G-quartet fragment (Kd = 7.4 nm). The ability to probe ligand surfaces in this manner is a powerful tool in the development of new therapeutic reagents with improved pharmacologic properties. The SOMAmer·IL-6 structure also expands our understanding of the diverse structural motifs achievable with modified nucleic acid libraries and elucidates the nature with which these unique ligands interact with their protein targets.
Organizational Affiliation:
From the SomaLogic, Inc., Boulder, Colorado 80301.