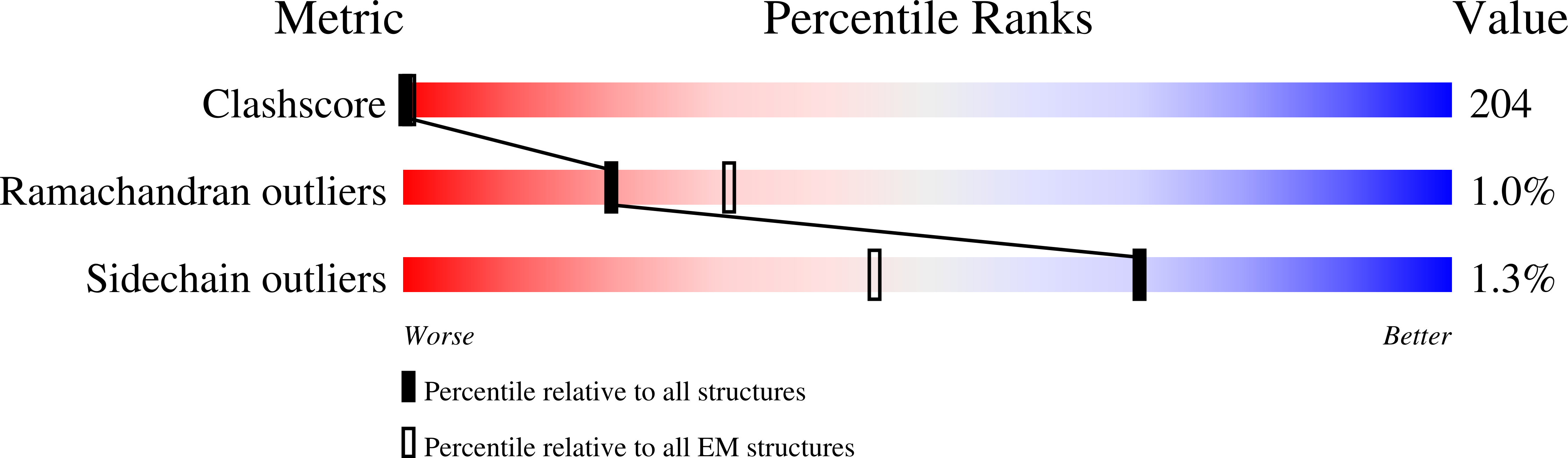Three-Dimensional Reconstruction of Intact Human Integrin Alphaiibbeta3; New Implications for Activation-Dependent Ligand Binding.
Choi, W.S., Rice, W.J., Stokes, D.L., Coller, B.S.(2013) Blood 122: 4165
- PubMed: 24136164
- DOI: https://doi.org/10.1182/blood-2013-04-499194
- Primary Citation of Related Structures:
4CAK - PubMed Abstract:
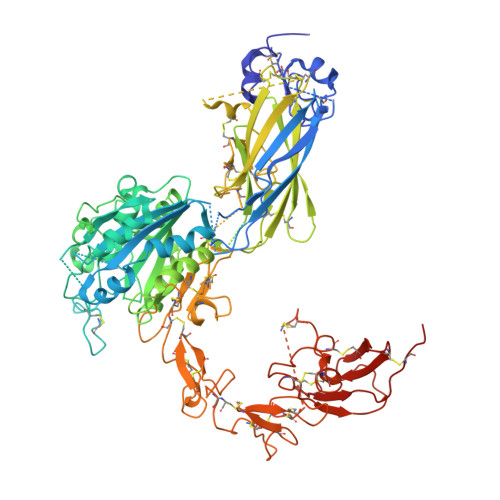
Integrin αIIbβ3 plays a central role in hemostasis and thrombosis. We provide the first 3-dimensional reconstruction of intact purified αIIbβ3 in a nanodisc lipid bilayer. Unlike previous models, it shows that the ligand-binding head domain is on top, pointing away from the membrane. Moreover, unlike the crystal structure of the recombinant ectodomain, the lower legs are not parallel, straight, and adjacent. Rather, the αIIb lower leg is bent between the calf-1 and calf-2 domains and the β3 Integrin-Epidermal Growth Factor (I-EGF) 2 to 4 domains are freely coiled rather than in a cleft between the β3 headpiece and the αIIb lower leg. Our data indicate an important role for the region that links the distal calf-2 and β-tail domains to their respective transmembrane (TM) domains in transmitting the conformational changes in the TM domains associated with inside-out activation.
Organizational Affiliation:
Laboratory of Blood and Vascular Biology, Rockefeller University, New York, NY;