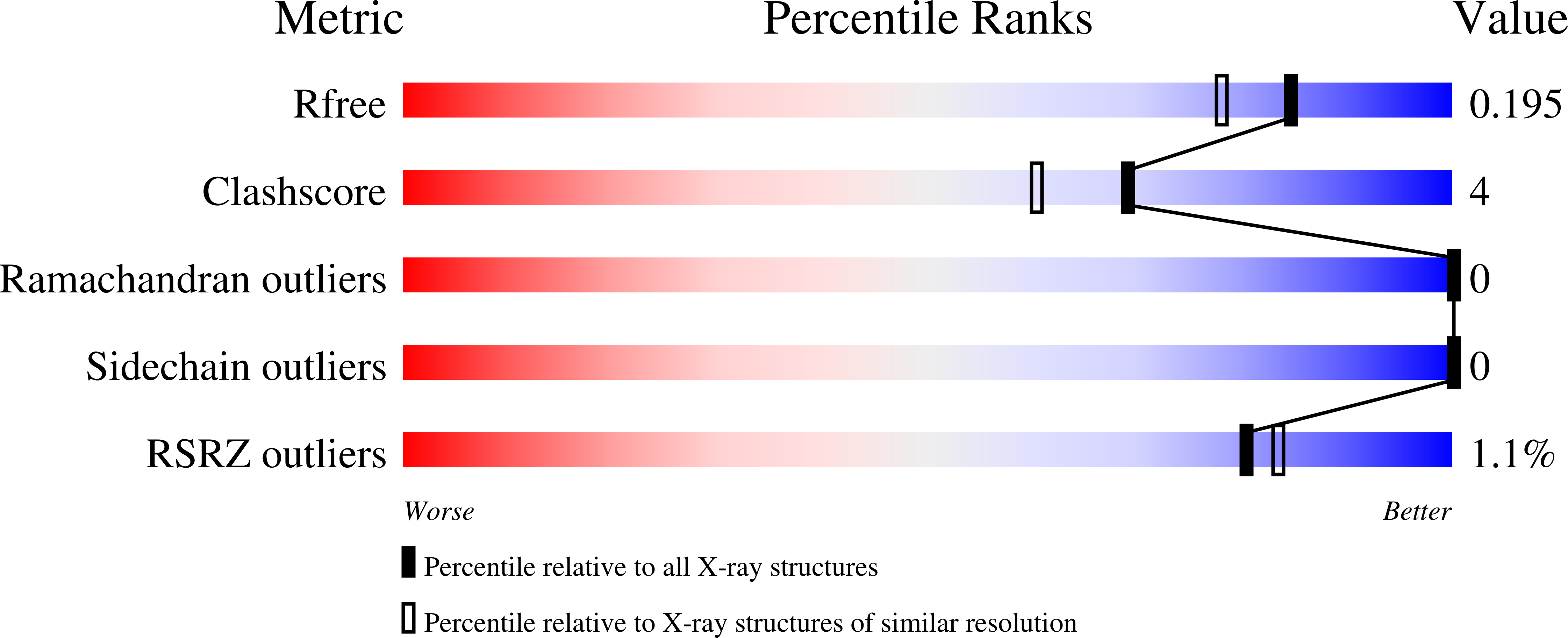
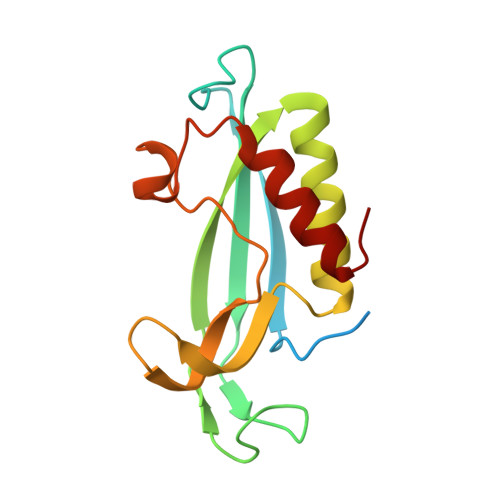
Fold of the conserved DTC domain in Deltex proteins.
Obiero, J., Walker, J.R., Dhe-Paganon, S.(2012) Proteins 80: 1495-1499
- PubMed: 22411408
- DOI: https://doi.org/10.1002/prot.24054
- Primary Citation of Related Structures:
3PG6 - PubMed Abstract:
Human Deltex 3-like (DTX3L) is a member of the Deltex family of proteins. Initially identified as a B-lymphoma and BAL-associated protein, DTX3L is an E3 ligase that regulates subcellular localization of its partner protein, BAL, by a dynamic nucleocytoplasmic trafficking mechanism. Unlike other members of the Deltex family of proteins, DTX3L lacks the highly basic N-terminal motif and the central proline-rich motif present in other Deltex proteins, and instead contains other unique N-terminal domains. The C-terminal domains are, however, homologous with other members of the Deltex family of proteins; these include a RING domain and a previously unidentified C-terminal domain. In this study, we report the high-resolution crystal structure of this previously uncharacterized C-terminal domain of human DTX3L, which we term the Deltex C-terminal domain.
Organizational Affiliation:
Structural Genomics Consortium, University of Toronto, Toronto, Ontario, Canada.