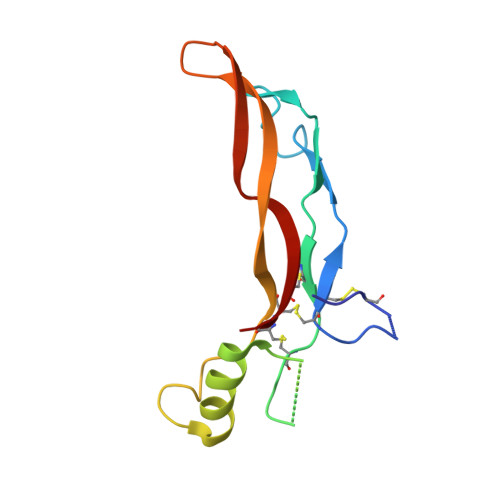
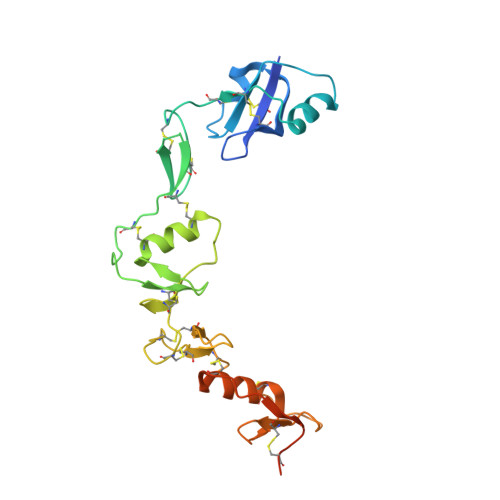
The structure of FSTL3.activin A complex. Differential binding of N-terminal domains influences follistatin-type antagonist specificity.
Stamler, R., Keutmann, H.T., Sidis, Y., Kattamuri, C., Schneyer, A., Thompson, T.B.(2008) J Biol Chem 283: 32831-32838
- PubMed: 18768470
- DOI: https://doi.org/10.1074/jbc.M801266200
- Primary Citation of Related Structures:
3B4V - PubMed Abstract:
Transforming growth factor beta family ligands are neutralized by a number of structurally divergent antagonists. Follistatin-type antagonists, which include splice variants of follistatin (FS288 and FS315) and follistatin-like 3 (FSTL3), have high affinity for activin A but differ in their affinity for other ligands, particularly bone morphogenetic proteins. To understand the structural basis for ligand specificity within FS-type antagonists, we determined the x-ray structure of activin A in complex with FSTL3 to a resolution of 2.5 A. Similar to the previously resolved FS.activin A structures, the ligand is encircled by two antagonist molecules blocking all ligand receptor-binding sites. Recently, the significance of the FS N-terminal domain interaction at the ligand type I receptor site has been questioned; however, our data show that for FSTL3, the N-terminal domain forms a more intimate contact with activin A, implying that this interaction is stronger than that for FS. Furthermore, binding studies revealed that replacing the FSTL3 N-terminal domain with the corresponding FS domain considerably lowers activin A affinity. Therefore, both structural and biochemical evidence support a significant interaction of the N-terminal domain of FSTL3 with activin A. In addition, structural comparisons with bone morphogenetic proteins suggest that the interface where the N-terminal domain binds may be the key site for determining FS-type antagonist specificity.
Organizational Affiliation:
Department of Molecular Genetics, Biochemistry and Microbiology, University of Cincinnati Medical Sciences Building, Cincinnati, Ohio 45267, USA.