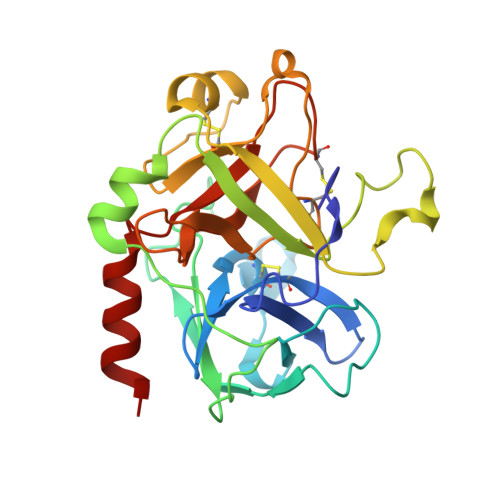
Crystal structure of thrombin in complex with s-variegin: insights of a novel mechanism of inhibition and design of tunable thrombin inhibitors
Koh, C.Y., Kumar, S., Kazimirova, M., Nuttall, P.A., Radhakrishnan, U.P., Kim, S., Jagadeeswaran, P., Imamura, T., Mizuguchi, J., Iwanaga, S., Swaminathan, K., Kini, R.M.(2011) PLoS One 6: e26367-e26367
- PubMed: 22053189
- DOI: https://doi.org/10.1371/journal.pone.0026367
- Primary Citation of Related Structures:
3B23 - PubMed Abstract:
The inhibition of thrombin is one of the important treatments of pathological blood clot formation. Variegin, isolated from the tropical bont tick, is a novel molecule exhibiting a unique 'two-modes' inhibitory property on thrombin active site (competitive before cleavage, noncompetitive after cleavage). For the better understanding of its function, we have determined the crystal structure of the human α-thrombin:synthetic-variegin complex at 2.4 Å resolution. The structure reveals a new mechanism of thrombin inhibition by disrupting the charge relay system. Based on the structure, we have designed 17 variegin variants, differing in potency, kinetics and mechanism of inhibition. The most active variant is about 70 times more potent than the FDA-approved peptidic thrombin inhibitor, hirulog-1/bivalirudin. In vivo antithrombotic effects of the variegin variants correlate well with their in vitro affinities for thrombin. Our results encourage that variegin and the variants show strong potential for the development of tunable anticoagulants.
Organizational Affiliation:
Department of Biological Sciences, National University of Singapore, Singapore, Singapore.