Diversity of bisubstrate binding modes of adenosine analogue-oligoarginine conjugates in protein kinase a and implications for protein substrate interactions.
Pflug, A., Rogozina, J., Lavogina, D., Enkvist, E., Uri, A., Engh, R.A., Bossemeyer, D.(2010) J Mol Biol 403: 66-77
- PubMed: 20732331
- DOI: https://doi.org/10.1016/j.jmb.2010.08.028
- Primary Citation of Related Structures:
3AG9, 3AGL, 3AGM - PubMed Abstract:
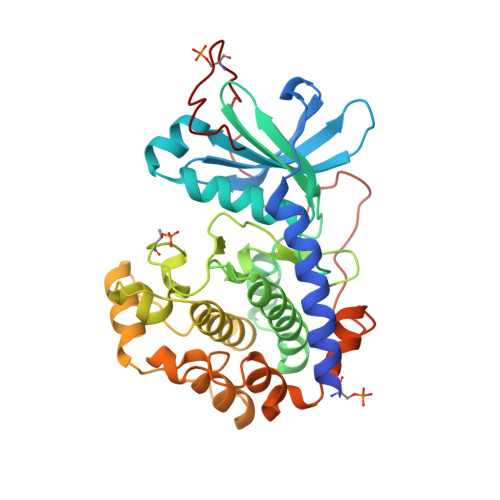
Crystal structures of the catalytic subunit α of cAMP-dependent protein kinase (PKAc) with three adenosine analogue-oligoarginine conjugates (ARCs) are presented. The rationally designed ARCs include moieties that, in combination, target both the ATP- and the peptide-substrate-binding sites of PKAc, thereby taking advantage of high-affinity binding interactions offered by the ATP site while utilizing an additional mechanism for target specificity via binding to the peptide substrate site. The crystal structures demonstrate that, in accord with the previously reported bisubstrate character of ARCs, the inhibitors occupy both binding sites of PKAc. Further, they show new binding modes that may also apply to natural protein substrates of PKAc, which have not been revealed by previous crystallographic studies. The crystal structures described here contribute to the understanding of the substrate-binding patterns of PKAc and should also facilitate the design of inhibitors targeting PKAc and related protein kinases.
Organizational Affiliation:
Norwegian Structural Biology Centre, Department of Chemistry, University of Tromsø, N-9037 Tromsø, Norway. Electronic address: alexander.pflug@uit.no.