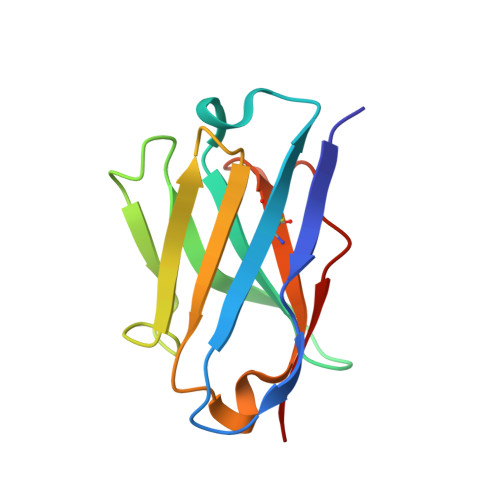
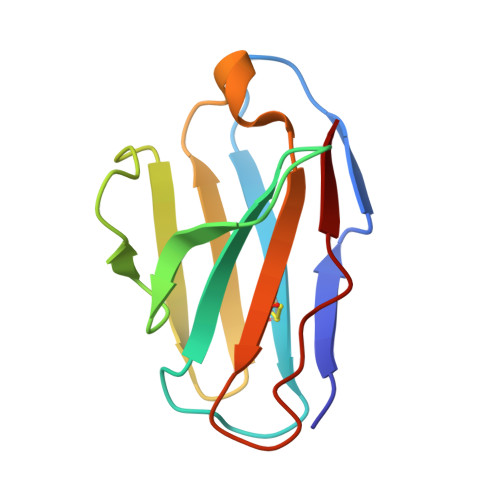
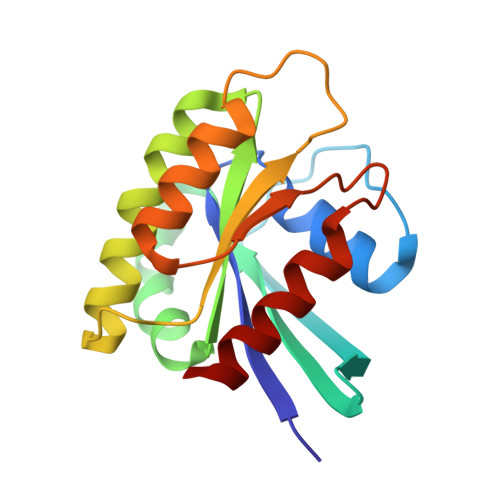
Tumour Prevention by a Single Antibody Domain Targeting the Interaction of Signal Transduction Proteins with Ras.
Tanaka, T., Williams, R.L., Rabbitts, T.H.(2007) EMBO J 26: 3250
- PubMed: 17568777
- DOI: https://doi.org/10.1038/sj.emboj.7601744
- Primary Citation of Related Structures:
2UZI - PubMed Abstract:
Many disease-related processes occur via protein complexes that are considered undruggable with small molecules. An example is RAS, which is frequently mutated in cancer and contributes to initiation and maintenance of the disease by constitutive signal transduction through protein interaction with effector proteins, like PI3K, RAF and RALGDS. Such protein interactions are therefore significant targets for therapy. We describe a single immunoglobulin variable region domain that specifically binds to activated GTP-bound RAS and prevents RAS-dependent tumorigenesis in a mouse model. The crystal structure of the immunoglobulin-RAS complex shows that the variable region competitively binds to the conformationally variant regions of RAS, where its signalling effector molecules interact. This allows the plasma membrane targeted single domain intrabody to inhibit signalling by mutant RAS. This mode of action is a novel advance to directly interfere with oncogenic RAS function in human cancer and shows a universally applicable approach to develop macromolecules to combat cancer. In addition, this method illustrates a general means for interfering with protein interactions that are commonly considered intractable as conventional drug targets.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Hills Road, Cambridge, UK.