Interconversion between two unrelated protein folds in the lymphotactin native state
Tuinstra, R.L., Peterson, F.C., Kutlesa, S., Elgin, E.S., Kron, M.A., Volkman, B.F.(2008) Proc Natl Acad Sci U S A 105: 5057-5062
- PubMed: 18364395
- DOI: https://doi.org/10.1073/pnas.0709518105
- Primary Citation of Related Structures:
2JP1 - PubMed Abstract:
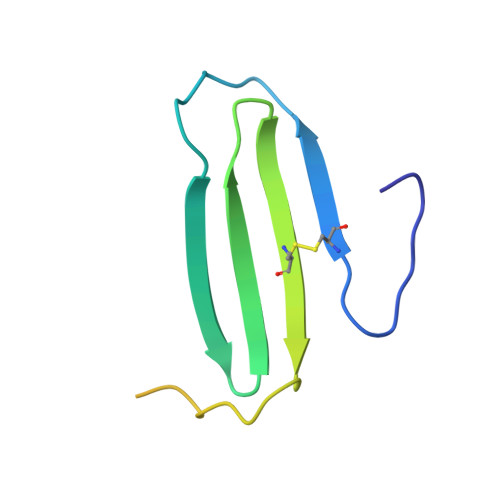
Proteins often have multiple functional states, which might not always be accommodated by a single fold. Lymphotactin (Ltn) adopts two distinct structures in equilibrium, one corresponding to the canonical chemokine fold consisting of a monomeric three-stranded beta-sheet and carboxyl-terminal helix. The second Ltn structure solved by NMR reveals a dimeric all-beta-sheet arrangement with no similarity to other known proteins. In physiological solution conditions, both structures are significantly populated and interconvert rapidly. Interconversion replaces long-range interactions that stabilize the chemokine fold with an entirely new set of tertiary and quaternary contacts. The chemokine-like Ltn conformation is a functional XCR1 agonist, but fails to bind heparin. In contrast, the alternative structure binds glycosaminoglycans with high affinity but fails to activate XCR1. Because each structural species displays only one of the two functional properties essential for activity in vivo, the conformational equilibrium is likely to be essential for the biological activity of lymphotactin. These results demonstrate that the functional repertoire and regulation of a single naturally occurring amino acid sequence can be expanded by access to a set of highly dissimilar native-state structures.
Organizational Affiliation:
Departments of Biochemistry and Medicine and Biotechnology and Bioengineering Center, Medical College of Wisconsin, Milwaukee, WI 53226, USA