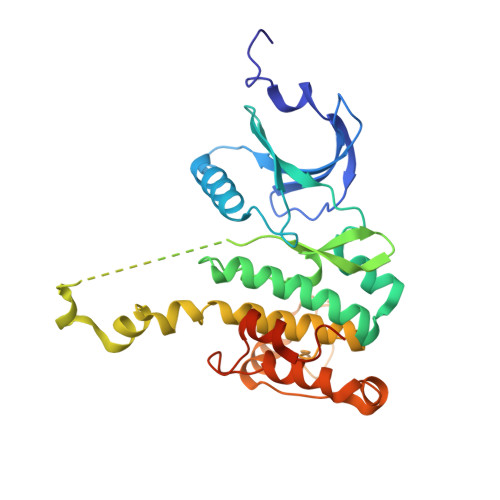
Crystal structures of the Mnk2 kinase domain reveal an inhibitory conformation and a zinc binding site.
Jauch, R., Jakel, S., Netter, C., Schreiter, K., Aicher, B., Jackle, H., Wahl, M.C.(2005) Structure 13: 1559-1568
- PubMed: 16216586
- DOI: https://doi.org/10.1016/j.str.2005.07.013
- Primary Citation of Related Structures:
2AC3, 2AC5 - PubMed Abstract:
Human mitogen-activated protein kinases (MAPK)-interacting kinases 1 and 2 (Mnk1 and Mnk2) target the translational machinery by phosphorylation of the eukaryotic initiation factor 4E (eIF4E). Here, we present the 2.1 A crystal structure of a nonphosphorylated Mnk2 fragment that encompasses the kinase domain. The results show Mnk-specific features such as a zinc binding motif and an atypical open conformation of the activation segment. In addition, the ATP binding pocket contains an Asp-Phe-Asp (DFD) in place of the canonical magnesium binding Asp-Phe-Gly (DFG) motif. The phenylalanine of this motif sticks into the ATP binding pocket and blocks ATP binding as observed with inhibitor bound and, thus, inactive p38 kinase. Replacement of the DFD by the canonical DFG motif affects the conformation of Mnk2, but not ATP binding and kinase activity. The results suggest that the ATP binding pocket and the activation segment of Mnk2 require conformational switches to provide kinase activity.
Organizational Affiliation:
Max-Planck-Institut für Biophysikalische Chemie, Abteilung Molekulare Entwicklungsbiologie, Am Fassberg 11, 37077 Göttingen, Germany.