Solution structure of the CS domain of human USP19
Nakanishi, T., Tochio, N., Koshiba, S., Inoue, M., Kigawa, T., Yokoyama, S.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ubiquitin carboxyl-terminal hydrolase 19 | 134 | Homo sapiens | Mutation(s): 0 Gene Names: KAZUSA cDNA hk08201 EC: 3.1.2.15 (PDB Primary Data), 3.4.19.12 (UniProt) | 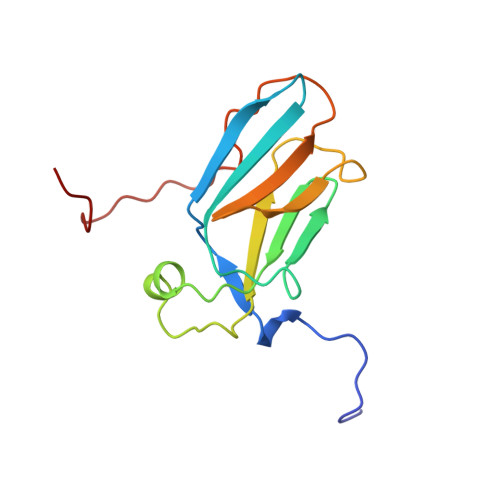 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O94966 (Homo sapiens) Explore O94966 Go to UniProtKB: O94966 | |||||
PHAROS: O94966 GTEx: ENSG00000172046 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O94966 | ||||
Sequence AnnotationsExpand | |||||
| |||||