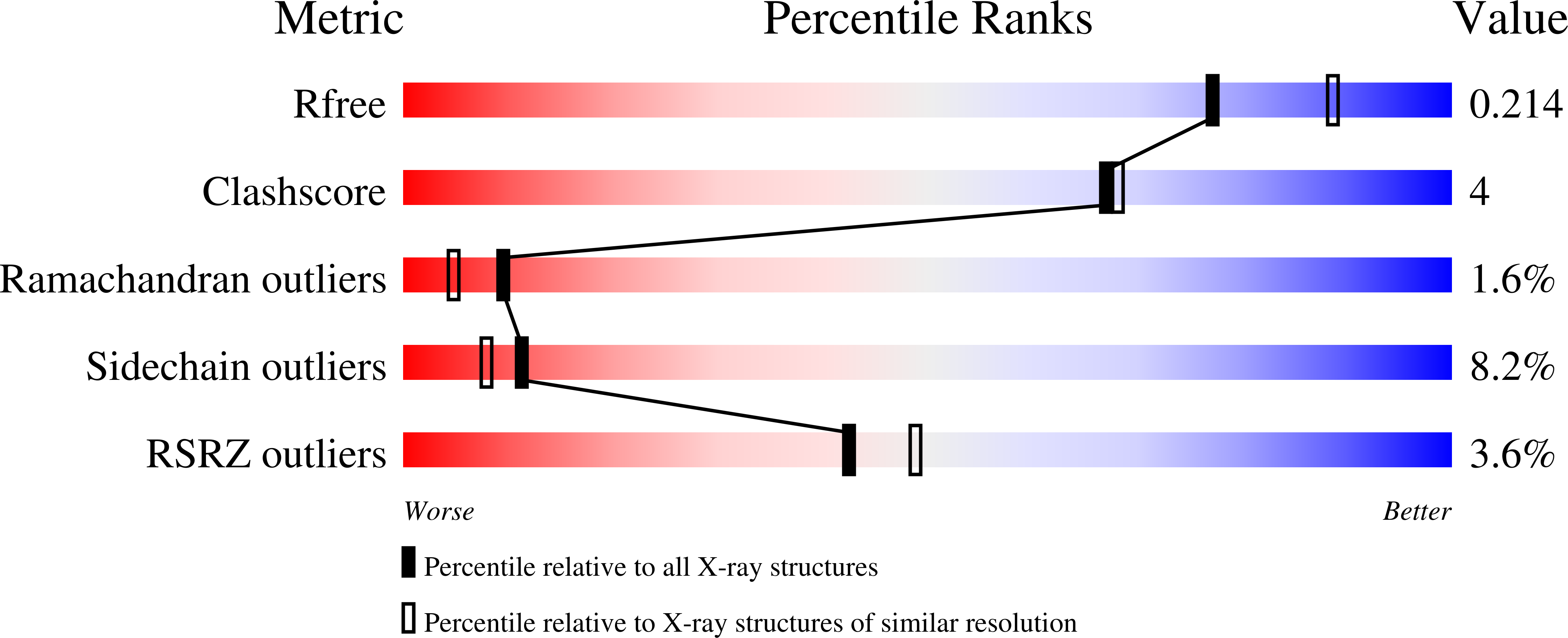
Substrate-induced Conformational Changes in Human UMP/CMP Kinase.
Sekulic, N., Ort, S., Konrad, M., Lavie, A.(2004) J Biol Chem 279: 33882-33889
- PubMed: 15163660
- DOI: https://doi.org/10.1074/jbc.M401989200
- Primary Citation of Related Structures:
1TEV - PubMed Abstract:
Human UMP/CMP kinase plays a crucial role in supplying precursors for nucleic acid synthesis by catalyzing the conversion of UMP, CMP, and dCMP into their diphosphate form. In addition, this kinase is an essential component of the activation cascade of medicinally relevant nucleoside analog prodrugs such as AraC, gemcitabine, and ddC. During the catalytic cycle the enzyme undergoes large conformational changes from open in the absence of substrates to closed in the presence of both phosphoryl donor and phosphoryl acceptor. Here we report the crystal structure of the substrate-free, open form of human UMP/CMP kinase. Comparison of the open structure with the closed state previously reported for the similar Dictyostelium discoideum UMP/CMP kinase reveals the conformational changes that occur upon substrate binding. We observe a classic example of induced fit where substrate-induced conformational changes in hinge residues result in rigid body movements of functional domains to form the catalytically competent state. In addition, a homology model of the human enzyme in the closed state based on the structure of D. discoideum UMP/CMP kinase aids to rationalize the substrate specificity of the human enzyme.
Organizational Affiliation:
University of Illinois at Chicago, Department of Biochemistry and Molecular Genetics, Chicago, Illinois 60607, USA.