Molecular Roots of Degenerate Specificity in Syntenin'S Pdz2 Domain: Reassessment of the Pdz Recognition Paradigm
Kang, B.S., Cooper, D.R., Devedjiev, Y., Derewenda, U., Derewenda, Z.S.(2003) Structure 11: 845
- PubMed: 12842047
- DOI: https://doi.org/10.1016/s0969-2126(03)00125-4
- Primary Citation of Related Structures:
1NTE, 1OBX, 1OBY, 1OBZ - PubMed Abstract:
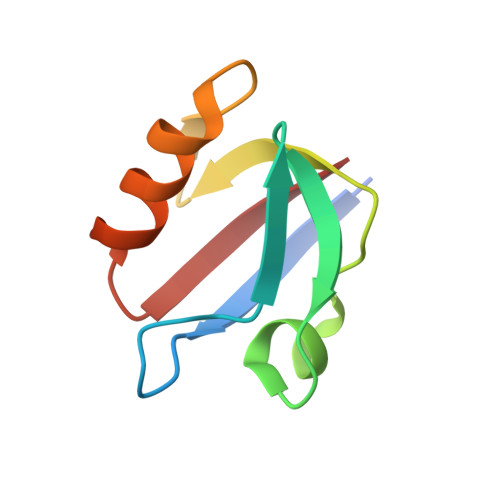
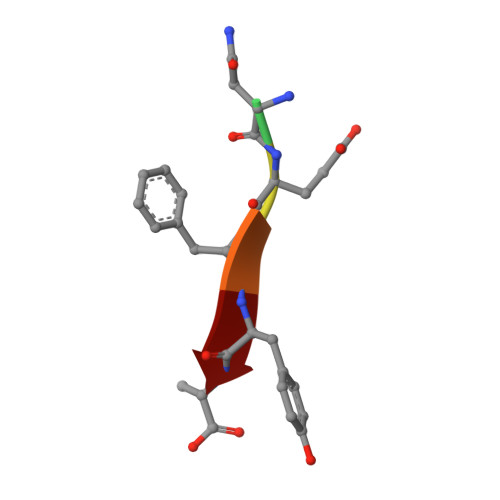
Crystal structures of the PDZ2 domain of the scaffolding protein syntenin, both unbound and in complexes with peptides derived from C termini of IL5 receptor (alpha chain) and syndecan, reveal the molecular roots of syntenin's degenerate specificity. Three distinct binding sites (S(0), S(-1), and S(-2)), with affinities for hydrophobic side chains, function in a combinatorial way: S(-1) and S(-2) act together to bind syndecan, while S(0) and S(-1) are involved in the binding of IL5Ralpha. Neither mode of interaction is consistent with the prior classification scheme, which defined the IL5Ralpha interaction as class I (-S/T-X-phi) and the syndecan interaction as class II (-phi-X-phi). These results, in conjunction with other emerging structural data on PDZ domains, call for a revision of their classification and of the existing model of their mechanism.
Organizational Affiliation:
Department of Molecular Physiology and Biological Physics, The Cancer Center, University of Virginia, Charlottesville, VA 22908, USA.