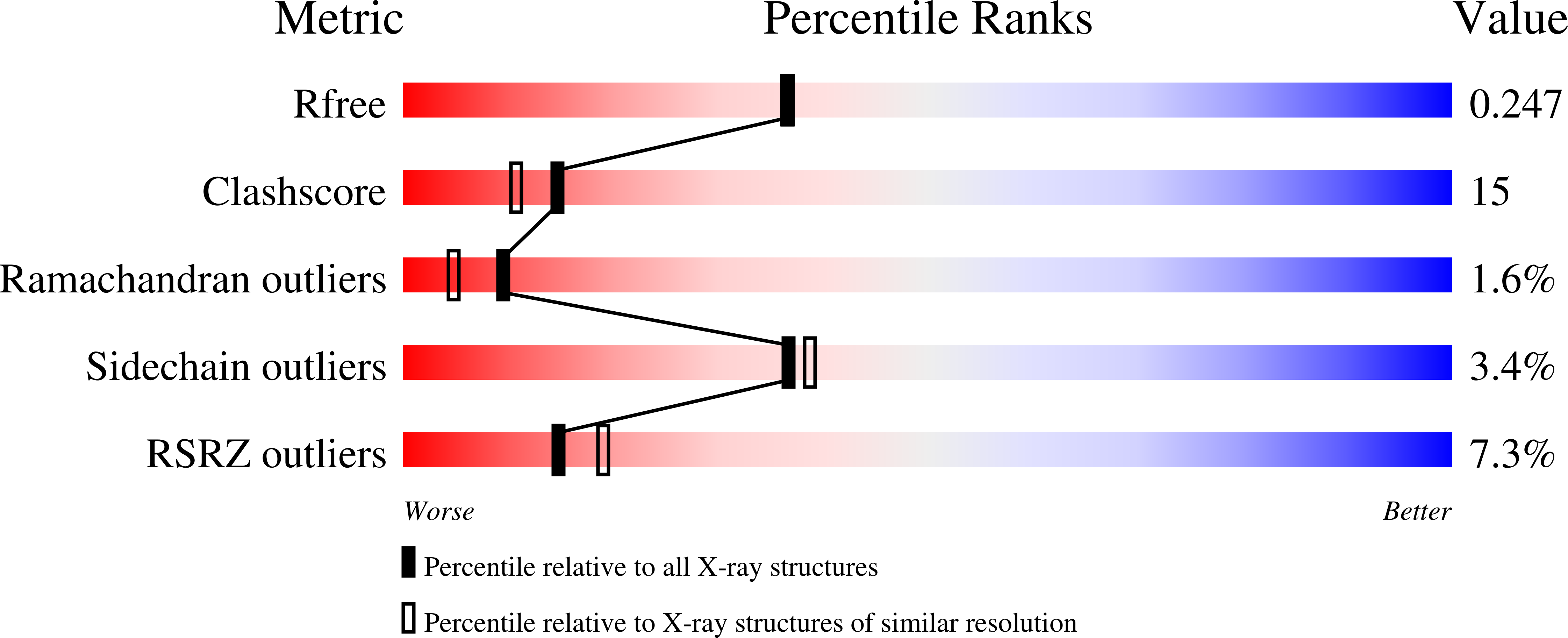
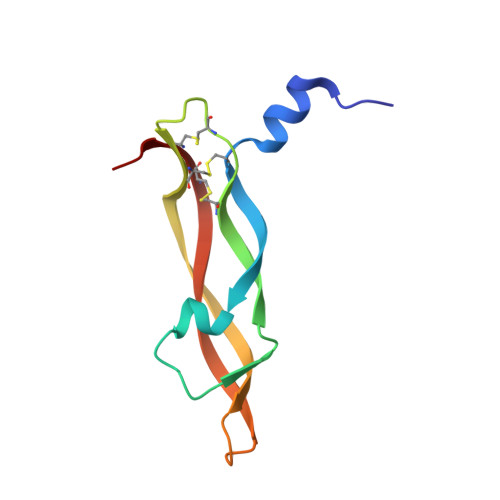
The cystine knot promotes folding and not thermodynamic stability in vascular endothelial growth factor
Muller, Y.A., Heiring, C., Misselwitz, R., Welfle, K., Welfle, H.(2002) J Biol Chem 277: 43410-43416
- PubMed: 12207021
- DOI: https://doi.org/10.1074/jbc.M206438200
- Primary Citation of Related Structures:
1MJV, 1MKG, 1MKK - PubMed Abstract:
Cystine knots consist of three intertwined disulfide bridges and are considered major determinants of protein stability in proteins in which they occur. We questioned this function and observed that removal of individual disulfide bridges in human vascular endothelial growth factor (VEGF) does not reduce its thermodynamic stability but reduces its unexpected high thermal stability of 108 degrees C by up to 40 degrees C. In wild-type VEGF (deltaG(u,25)(0) = 5.1 kcal.mol(-1)), the knot is responsible for a large entropic stabilization of TdeltaS(u,25)(0) = -39.3 kcal mol(-1), which is compensated for by a deltaH(u,25)(0) of -34.2 kcal mol(-1). In the disulfide-deficient mutants, this entropic stabilization disappears, but instead of a decrease, we observe an increase in the thermodynamic stability by about 2 kcal.mol(-1). A detailed crystallographic analysis of the mutant structures suggests a role of the cystine knot motif in protein folding rather than in the stabilization of the folded state. When assuming that the sequential order of the disulfide bridge formation is conserved between VEGF and glycoprotein alpha-subunit, the crystal structure of the mutant C61A-C104A, which deviates by a root mean square deviation of more than 2.2 A from wild-type VEGF, identifies a true folding intermediate of VEGF.
Organizational Affiliation:
Forschungsgruppe Kristallographie, Max-Delbrück-Centrum für Molekulare Medizin, Berlin, D-13092 Berlin, Germany. y.muller@sussex.ac.uk