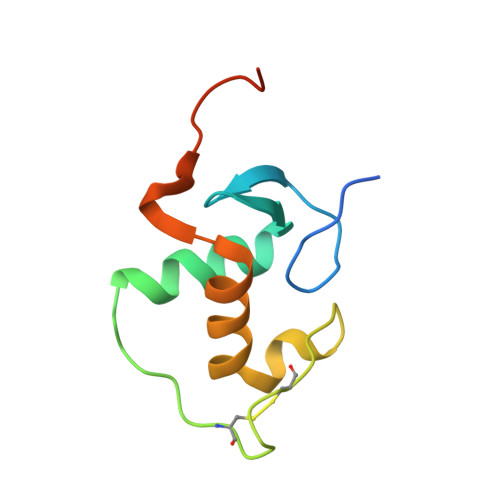
DNA Deformability as a Recognition Feature in the RevErb Response Element
Sierk, M.L., Zhao, Q., Rastinejad, F.(2001) Biochemistry 40: 12833-12843
- PubMed: 11669620
- DOI: https://doi.org/10.1021/bi011086r
- Primary Citation of Related Structures:
1GA5, 1HLZ - PubMed Abstract:
Most nuclear receptors recognize the same consensus hexameric sequence, AGGTCA. An important question has been how the various members of this transcription factor family distinguish identity features in these closely related DNA sites. We determined structures from several crystal forms of the RevErb-DNA complex and analyzed the patterns of protein-DNA interactions and DNA distortions. We found a significant and consistent DNA distortion at a TA step directly preceding the first consensus 5'-AGGTCA-3' recognition sequence. Importantly, while this base-pair sequence is associated with RevErb's high-affinity sites, there are no sequence-specific contacts formed with the protein. Our study shows that RevErb relies instead on the intrinsic geometry and flexibility of this TA site to make the required fit between the proteins' independent major groove and minor groove binding interactions, which occur on both sides of the TA step. Our findings extend the description of response element discrimination to include a role for sequence-dependent DNA deformations and suggest how other monomeric members of this superfamily, such as NGFI-B, SF-1, and ROR, could also recognize unique geometric features in their DNA targets.
Organizational Affiliation:
Department of Pharmacology, University of Virginia, Charlottesville, Virginia 22908-0735, USA.