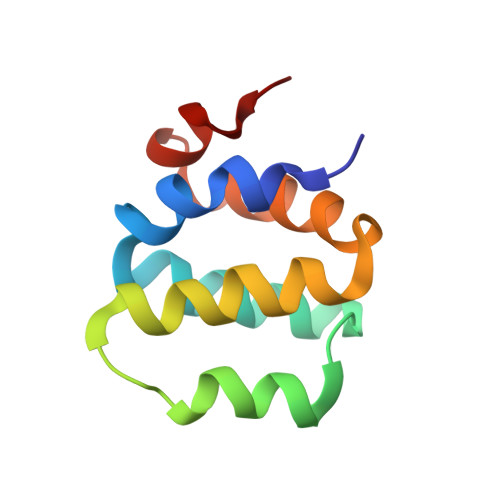
ICEBERG: a novel inhibitor of interleukin-1beta generation.
Humke, E.W., Shriver, S.K., Starovasnik, M.A., Fairbrother, W.J., Dixit, V.M.(2000) Cell 103: 99-111
- PubMed: 11051551
- DOI: https://doi.org/10.1016/s0092-8674(00)00108-2
- Primary Citation of Related Structures:
1DGN - PubMed Abstract:
ProIL-1beta is a proinflammatory cytokine that is proteolytically processed to its active form by caspase-1. Upon receipt of a proinflammatory stimulus, an upstream adaptor, RIP2, binds and oligomerizes caspase-1 zymogen, promoting its autoactivation. ICEBERG is a novel protein that inhibits generation of IL-1beta by interacting with caspase-1 and preventing its association with RIP2. ICEBERG is induced by proinflammatory stimuli, suggesting that it may be part of a negative feedback loop. Consistent with this, enforced retroviral expression of ICEBERG inhibits lipopolysaccharide-induced IL-1beta generation. The structure of ICEBERG reveals it to be a member of the death-domain-fold superfamily. The distribution of surface charge is complementary to the homologous prodomain of caspase-1, suggesting that charge-charge interactions mediate binding of ICEBERG to the prodomain of caspase-1.
Organizational Affiliation:
Department of Cellular and Molecular Biology, University of Michigan Medical School, Ann Arbor 48109, USA.