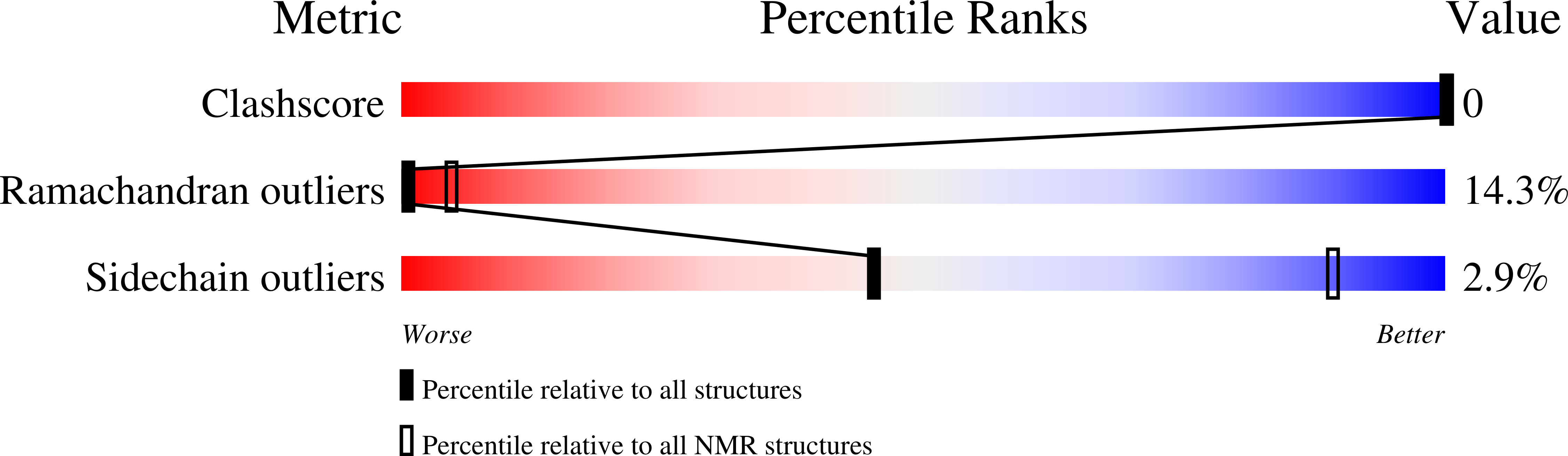Structure of antibody-bound peptides and retro-inverso analogues. A transferred nuclear Overhauser effect spectroscopy and molecular dynamics approach.
Phan-Chan-Du, A., Petit, M.C., Guichard, G., Briand, J.P., Muller, S., Cung, M.T.(2001) Biochemistry 40: 5720-5727
- PubMed: 11341837
- DOI: https://doi.org/10.1021/bi001151h
- Primary Citation of Related Structures:
1CS9, 1CT6, 1CVQ, 1CW8, 1CWZ - PubMed Abstract:
The three-dimensional structures of the two L-peptides, H-CGGIRGERA-OH, called L(A), and H-CGGIRGERG-OH, called L(G), corresponding or close to the IRGERA sequence present in the C-terminal region (residues 130-135) of histone H3, and their retro-inverso analogues HO-mAreGriGGC-NH2, called RI(mA), and HO-mGreGriGGC-NH2, called RI(mG), have been studied by two-dimensional 1H NMR and molecular dynamics calculations in association with a monoclonal antibody generated against L(A). At 25 degrees C, the affinity constants of the monoclonal antibody with respect to RI(mA) and RI(mG) were 75- and 270-fold higher than those measured with the homologous L(A) and L(G) peptides, respectively. Due to the spontaneous epimerization of the mA malonic residue, RI(mA) gave rise to two sets of resonances. With regard to the NH amide region, one set was similar to that for RI(mG) while the second was similar to those for the parent L-peptides L(A) and L(G). The antibody-bound conformations of the two couples of L- and retro-inverso peptides have been analyzed using molecular modeling calculations based on the transferred NOE interproton distances. Folded structures appeared in both cases with a type II' beta-turn in the parent GGIR sequence and a type I' beta-turn in the retro-inverso reGr sequence.
Organizational Affiliation:
Laboratoire de Chimie-Physique Macromoléculaire, UMR 7568 CNRS-INPL, Groupe ENSIC, 1 rue Grandville, B.P. 451, 54001 Nancy Cedex, France.