Structural insights into the mechanism of phosphate recognition and transport by XPR1.
Zhang, W., Chen, Y., Guan, Z., Wang, Y., Tang, M., Du, Z., Zhang, J., Cheng, M., Zuo, J., Liu, Y., Wang, Q., Liu, Y., Zhang, D., Yin, P., Ma, L., Liu, Z.(2025) Nat Commun 16: 18-18
- PubMed: 39747008
- DOI: https://doi.org/10.1038/s41467-024-55471-9
- Primary Citation of Related Structures:
9J4X, 9J51, 9J52, 9J53 - PubMed Abstract:
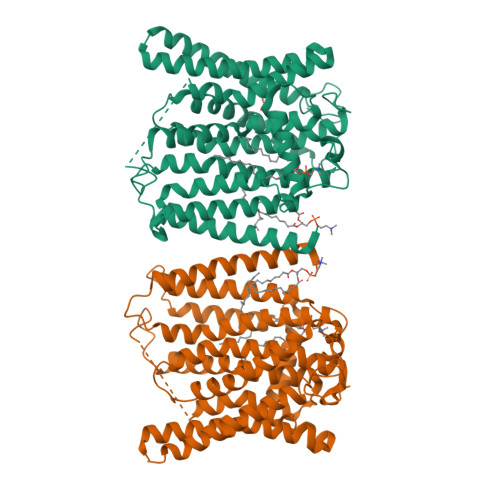
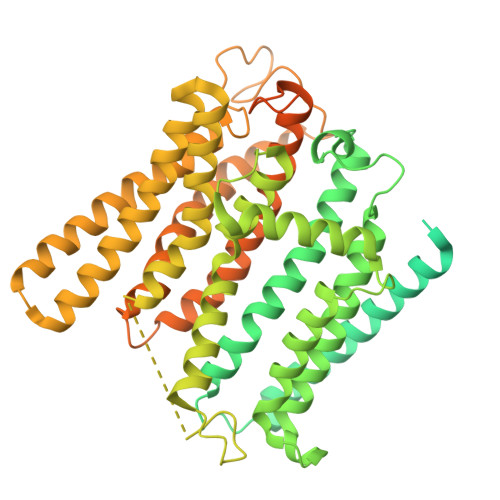
XPR1 is the sole protein known to transport inorganic phosphate (Pi) out of cells, a function conserved across species from yeast to mammals. Human XPR1 variants lead to cerebral calcium-phosphate deposition and primary familial brain calcification (PFBC), a hereditary neurodegenerative disorder. Here, we present the cryo-EM structure of human XPR1 in both its Pi-unbound and various Pi-bound states. XPR1 features 10 transmembrane α-helices forming an ion channel-like structure, with multiple Pi recognition sites along the channel. Pathogenic mutations in two arginine residues, which line the translocation channel, disrupt Pi transport. Molecular dynamics simulations reveal that Pi ion undergoes a stepwise transition through the sequential recognition sites during the transport process. Together with functional analyses, our results suggest that this sequential arrangement allows XPR1 to facilitate Pi ion passage via a "relay" process, and they establish a framework for the interpretation of disease-related mutations and for the development of future therapeutics.
Organizational Affiliation:
National Key Laboratory of Crop Genetic Improvement, Hubei Hongshan Laboratory, Huazhong Agricultural University, Wuhan, China.