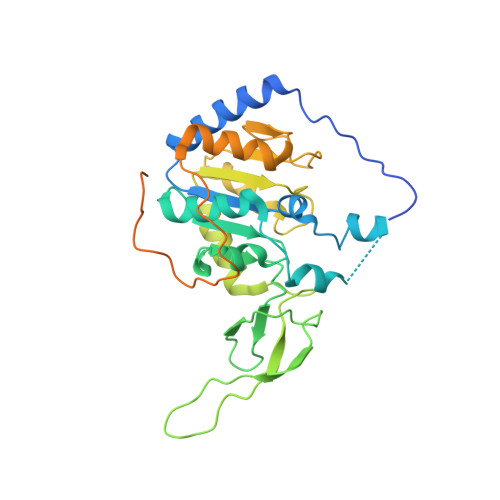
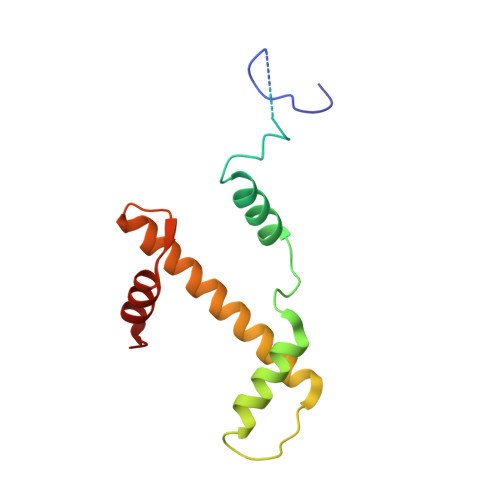
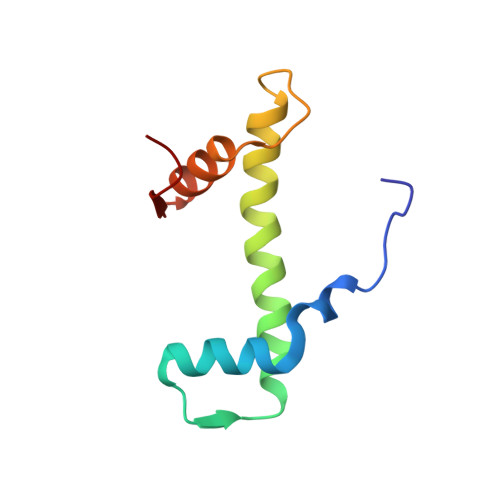
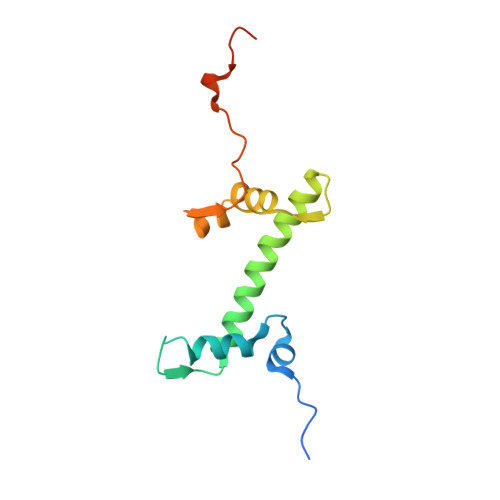
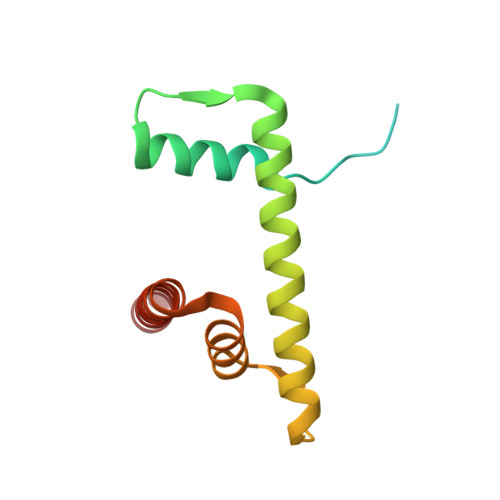
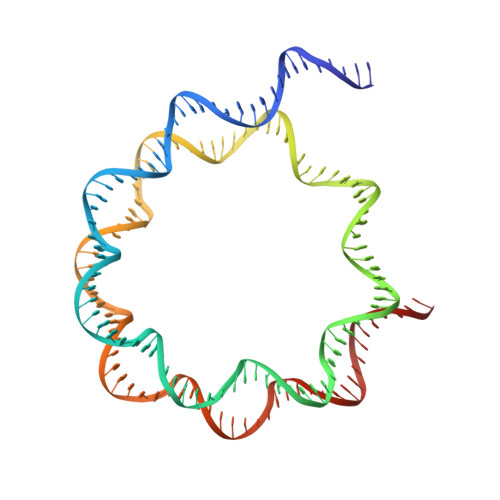
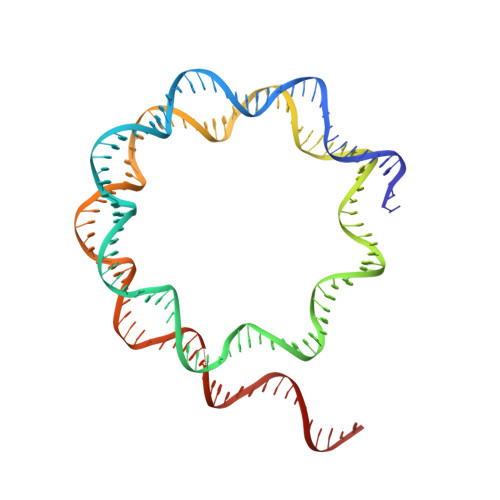
Cryo-EM structure of the human Sirtuin 6-nucleosome complex.
Chio, U.S., Rechiche, O., Bryll, A.R., Zhu, J., Leith, E.M., Feldman, J.L., Peterson, C.L., Tan, S., Armache, J.P.(2023) Sci Adv 9: eadf7586-eadf7586
- PubMed: 37058572
- DOI: https://doi.org/10.1126/sciadv.adf7586
- Primary Citation of Related Structures:
8G57 - PubMed Abstract:
Sirtuin 6 (SIRT6) is a multifaceted protein deacetylase/deacylase and a major target for small-molecule modulators of longevity and cancer. In the context of chromatin, SIRT6 removes acetyl groups from histone H3 in nucleosomes, but the molecular basis for its nucleosomal substrate preference is unknown. Our cryo-electron microscopy structure of human SIRT6 in complex with the nucleosome shows that the catalytic domain of SIRT6 pries DNA from the nucleosomal entry-exit site and exposes the histone H3 N-terminal helix, while the SIRT6 zinc-binding domain binds to the histone acidic patch using an arginine anchor. In addition, SIRT6 forms an inhibitory interaction with the C-terminal tail of histone H2A. The structure provides insights into how SIRT6 can deacetylate both H3 K9 and H3 K56.
Organizational Affiliation:
Center for Eukaryotic Gene Regulation, Department of Biochemistry and Molecular Biology, Pennsylvania State University, University Park, PA 16802, USA.