A fungal tolerance trait and selective inhibitors proffer HMG-CoA reductase as a herbicide mode-of-action.
Haywood, J., Breese, K.J., Zhang, J., Waters, M.T., Bond, C.S., Stubbs, K.A., Mylne, J.S.(2022) Nat Commun 13: 5563-5563
- PubMed: 36137996
- DOI: https://doi.org/10.1038/s41467-022-33185-0
- Primary Citation of Related Structures:
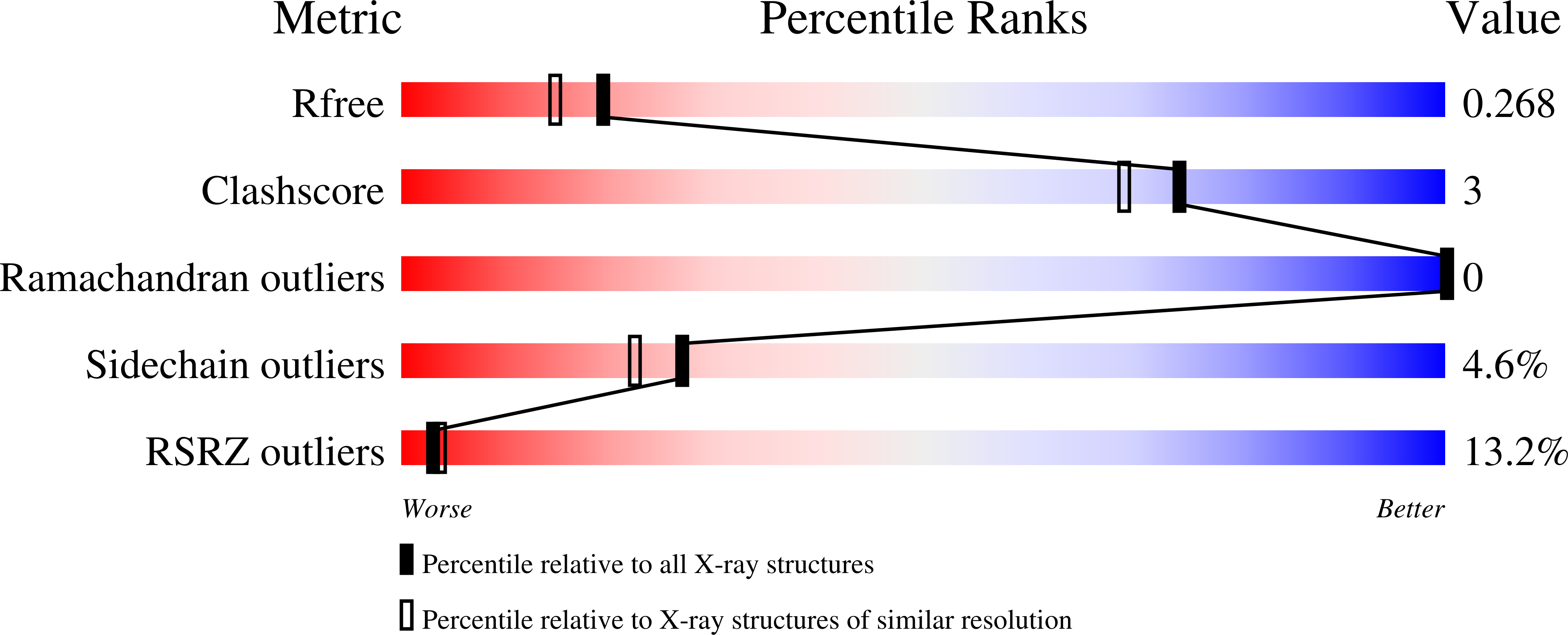
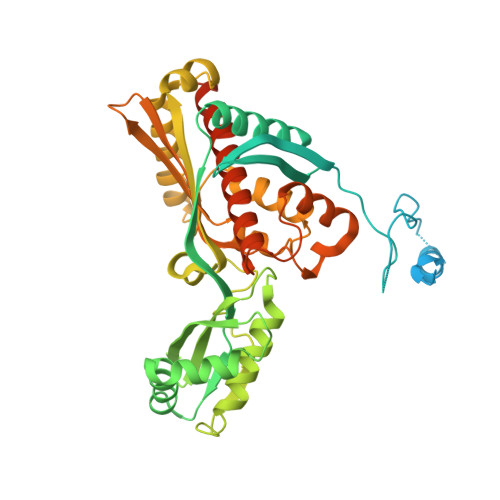
7ULI, 8ECG - PubMed Abstract:
Decades of intense herbicide use has led to resistance in weeds. Without innovative weed management practices and new herbicidal modes of action, the unabated rise of herbicide resistance will undoubtedly place further stress upon food security. HMGR (3-hydroxy-3-methylglutaryl-coenzyme A reductase) is the rate limiting enzyme of the eukaryotic mevalonate pathway successfully targeted by statins to treat hypercholesterolemia in humans. As HMGR inhibitors have been shown to be herbicidal, HMGR could represent a mode of action target for the development of herbicides. Here, we present the crystal structure of a HMGR from Arabidopsis thaliana (AtHMG1) which exhibits a wider active site than previously determined structures from different species. This plant conserved feature enables the rational design of specific HMGR inhibitors and we develop a tolerance trait through sequence analysis of fungal gene clusters. These results suggest HMGR to be a viable herbicide target modifiable to provide a tolerance trait.
Organizational Affiliation:
Centre for Crop and Disease Management, School of Molecular and Life Sciences, Curtin University, Bentley, Perth, WA, 6102, Australia. joel.haywood@curtin.edu.au.