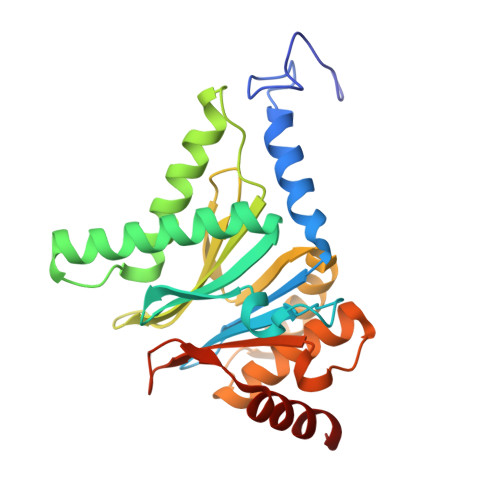
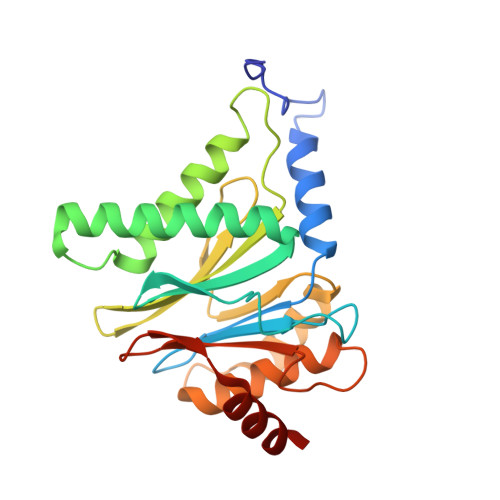
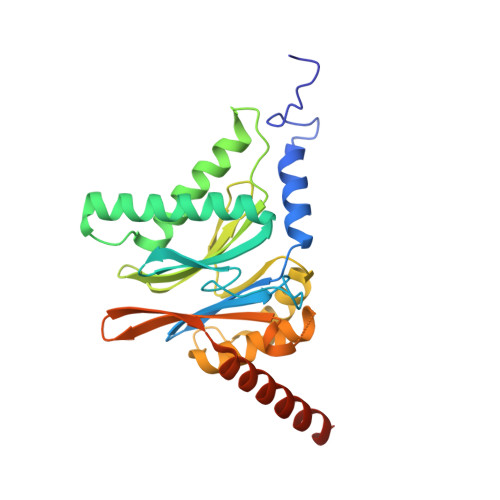
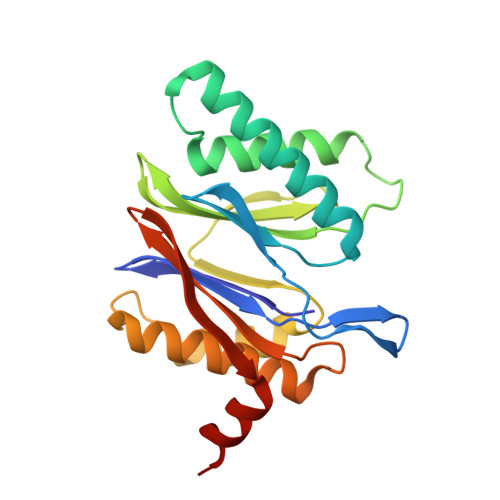
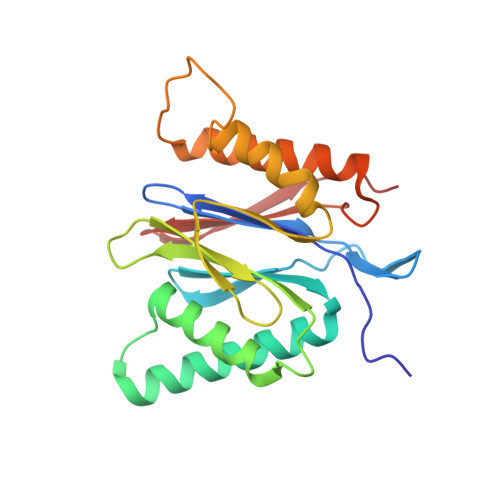
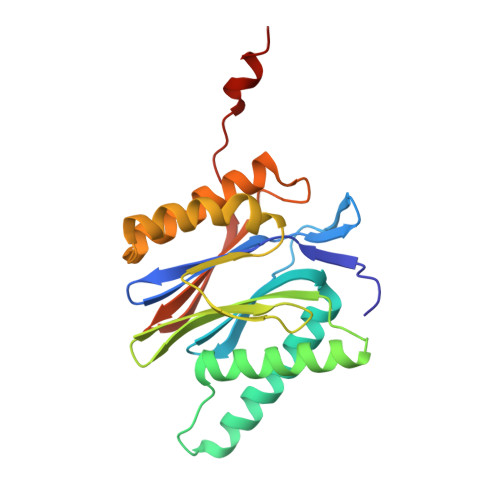
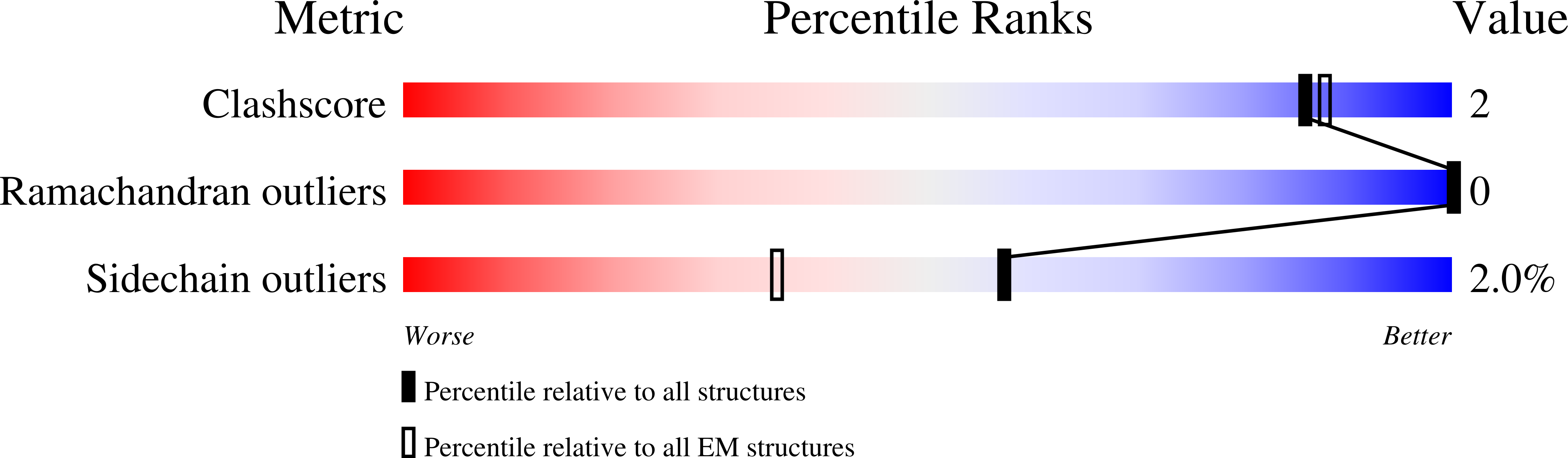Structures revealing mechanisms of resistance and collateral sensitivity of Plasmodium falciparum to proteasome inhibitors.
Hsu, H.C., Li, D., Zhan, W., Ye, J., Liu, Y.J., Leung, A., Qin, J., Crespo, B., Gamo, F.J., Zhang, H., Cui, L., Roth, A., Kirkman, L.A., Li, H., Lin, G.(2023) Nat Commun 14: 8302-8302
- PubMed: 38097652
- DOI: https://doi.org/10.1038/s41467-023-44077-2
- Primary Citation of Related Structures:
8G6E, 8G6F, 8UD9 - PubMed Abstract:
The proteasome of the malaria parasite Plasmodium falciparum (Pf20S) is an advantageous drug target because its inhibition kills P. falciparum in multiple stages of its life cycle and synergizes with artemisinins. We recently developed a macrocyclic peptide, TDI-8304, that is highly selective for Pf20S over human proteasomes and is potent in vitro and in vivo against P. falciparum. A mutation in the Pf20S β6 subunit, A117D, confers resistance to TDI-8304, yet enhances both enzyme inhibition and anti-parasite activity of a tripeptide vinyl sulfone β2 inhibitor, WLW-vs. Here we present the high-resolution cryo-EM structures of Pf20S with TDI-8304, of human constitutive proteasome with TDI-8304, and of Pf20Sβ6 A117D with WLW-vs that give insights into the species selectivity of TDI-8304, resistance to it, and the collateral sensitivity associated with resistance, including that TDI-8304 binds β2 and β5 in wild type Pf20S as well as WLW-vs binds β2 and β5 in Pf20Sβ6 A117D . We further show that TDI-8304 kills P. falciparum as quickly as chloroquine and artemisinin and is active against P. cynomolgi at the liver stage. This increases interest in using these structures to facilitate the development of Pf20S inhibitors that target multiple proteasome subunits and limit the emergence of resistance.
Organizational Affiliation:
Department of Structural Biology, Van Andel Institute, 333 Bostwick Ave NE, Grand Rapids, MI, 49503, USA.