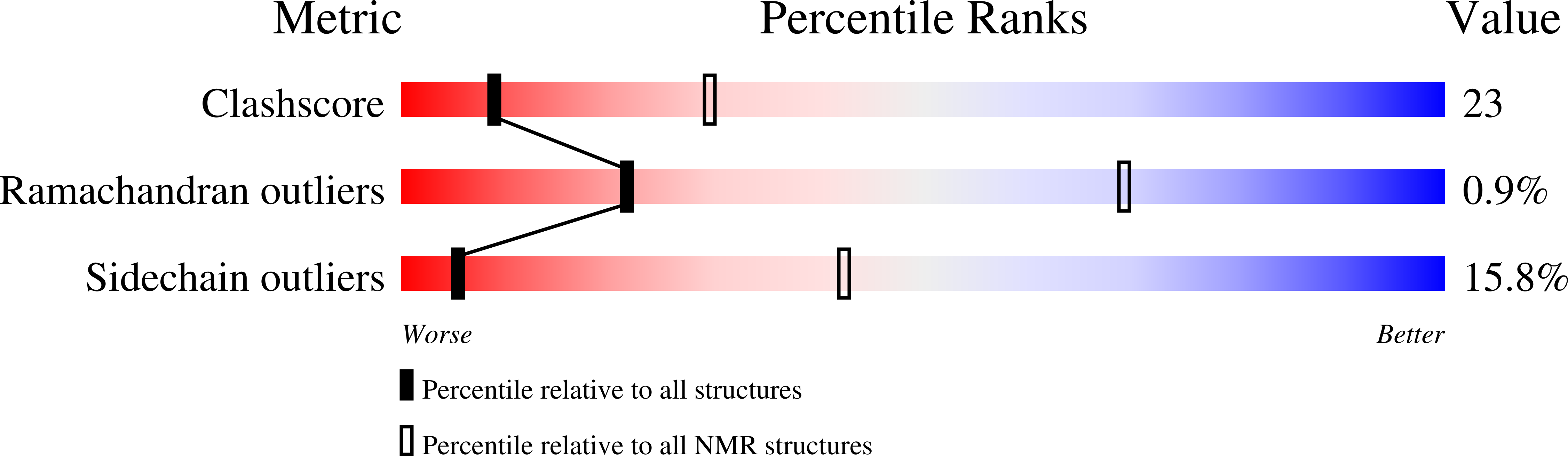Structural insights for selective disruption of Beclin 1 binding to Bcl-2.
Pan, Y.Z., Liang, Q., Tomchick, D.R., De Brabander, J.K., Rizo, J.(2023) Commun Biol 6: 1080-1080
- PubMed: 37875561
- DOI: https://doi.org/10.1038/s42003-023-05467-w
- Primary Citation of Related Structures:
8U27 - PubMed Abstract:
Stimulation of autophagy could provide powerful therapies for multiple diseases, including cancer and neurodegeneration. An attractive drug target for this purpose is Bcl-2, which inhibits autophagy by binding to the Beclin 1 BH3-domain. However, compounds that preclude Beclin 1/Bcl-2 binding might also induce apoptosis, which is inhibited by binding of Bcl-2 to BH3-domains of pro-apoptosis factors such as Bax. Here we describe the NMR structure of Bcl-2 bound to 35, a compound that we recently found to inhibit Beclin 1/Bcl-2 binding more potently than Bax/Bcl-2 binding. The structure shows that 35 binds at one end of the BH3-binding groove of Bcl-2. Interestingly, much of the 35-binding site is not involved in binding to Bcl-2 inhibitors described previously and mediates binding to Beclin 1 but not Bax. The structure suggests potential avenues to design compounds that disrupt Beclin 1/Bcl-2 binding and stimulate autophagy without inducing apoptosis.
Organizational Affiliation:
Department of Biophysics, University of Texas Southwestern Medical Center, Dallas, TX, 75390, USA.