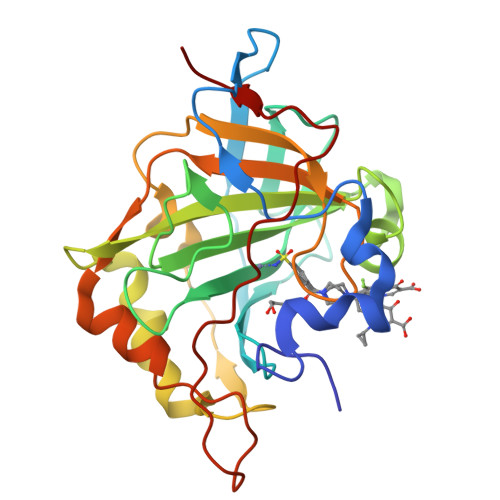
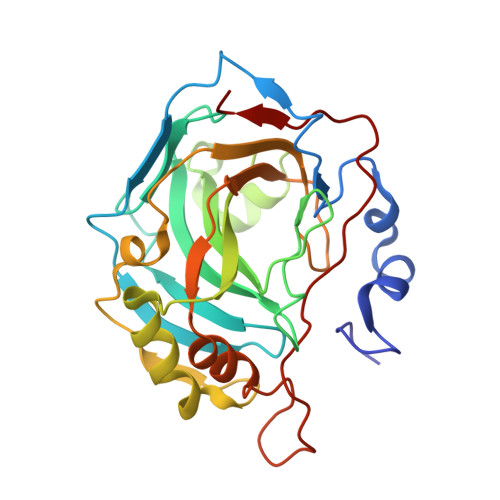
Inhibition of Pseudomonas aeruginosa Carbonic Anhydrases, Exploring Ciprofloxacin Functionalization Toward New Antibacterial Agents: An In-Depth Multidisciplinary Study.
Marinacci, B., D'Agostino, I., Angeli, A., Carradori, S., Melfi, F., Grande, R., Corsiani, M., Ferraroni, M., Agamennone, M., Tondo, A.R., Zara, S., Puca, V., Pellegrini, B., Vagaggini, C., Dreassi, E., Patrauchan, M.A., Capasso, C., Nicolotti, O., Carta, F., Supuran, C.T.(2024) J Med Chem 67: 19077-19102
- PubMed: 39453626
- DOI: https://doi.org/10.1021/acs.jmedchem.4c01555
- Primary Citation of Related Structures:
8OTP, 8OUB - PubMed Abstract:
Ciprofloxacin (CPX) is one of the most employed antibiotics in clinics to date. However, the rise of drug-resistant bacteria is dramatically impairing its efficacy, especially against life-threatening pathogens, such as Pseudomonas aeruginosa . This Gram-negative bacterium is an opportunistic pathogen, often infecting immuno-compromised patients with severe or fatal outcomes. The evidence of the possibility of exploiting Carbonic Anhydrase (CA, EC: 4.2.1.1) enzymes as pharmacological targets along with their role in P. aeruginosa virulence inspired the derivatization of CPX with peculiar CA-inhibiting chemotypes. Thus, a large library of CPX derivatives was synthesized and tested on a panel of bacterial CAs and human isoenzymes I and II. Selected derivatives were evaluated for antibacterial activity, revealing bactericidal and antibiofilm properties for some compounds. Importantly, promising preliminary absorption, distribution, metabolism, and excretion (ADME) properties in vitro were found and no cytotoxicity was detected for some representative compounds when tested in Galleria mellonella larvae.
Organizational Affiliation:
Department of Pharmacy, "G. d'Annunzio" University of Chieti-Pescara, 66100 Chieti, Italy.