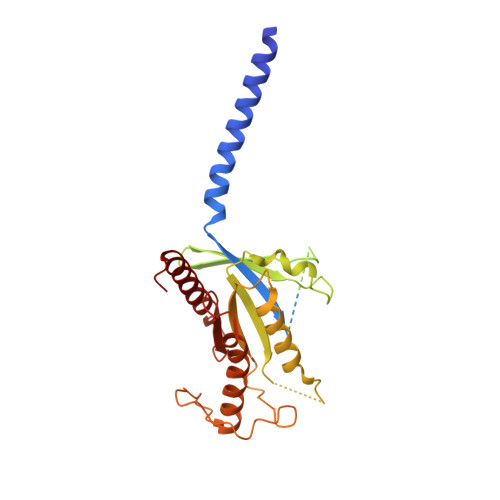
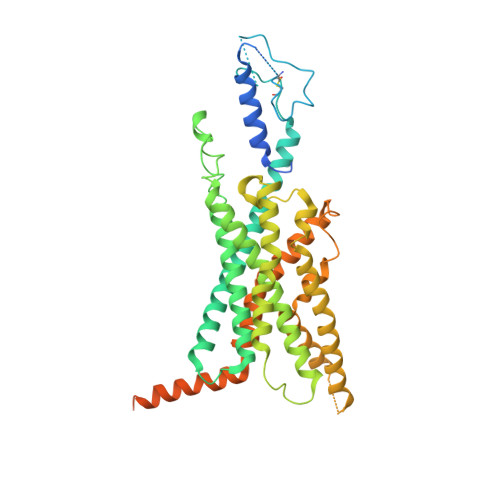
Negative allosteric modulation of the glucagon receptor by RAMP2.
Krishna Kumar, K., O'Brien, E.S., Habrian, C.H., Latorraca, N.R., Wang, H., Tuneew, I., Montabana, E., Marqusee, S., Hilger, D., Isacoff, E.Y., Mathiesen, J.M., Kobilka, B.K.(2023) Cell 186: 1465-1477.e18
- PubMed: 37001505
- DOI: https://doi.org/10.1016/j.cell.2023.02.028
- Primary Citation of Related Structures:
8FU6 - PubMed Abstract:
Receptor activity-modifying proteins (RAMPs) modulate the activity of many Family B GPCRs. We show that RAMP2 directly interacts with the glucagon receptor (GCGR), a Family B GPCR responsible for blood sugar homeostasis, and broadly inhibits receptor-induced downstream signaling. HDX-MS experiments demonstrate that RAMP2 enhances local flexibility in select locations in and near the receptor extracellular domain (ECD) and in the 6 th transmembrane helix, whereas smFRET experiments show that this ECD disorder results in the inhibition of active and intermediate states of the intracellular surface. We determined the cryo-EM structure of the GCGR-G s complex at 2.9 Å resolution in the presence of RAMP2. RAMP2 apparently does not interact with GCGR in an ordered manner; however, the receptor ECD is indeed largely disordered along with rearrangements of several intracellular hallmarks of activation. Our studies suggest that RAMP2 acts as a negative allosteric modulator of GCGR by enhancing conformational sampling of the ECD.
Organizational Affiliation:
Department of Molecular and Cellular Physiology, Stanford University School of Medicine, 279 Campus Drive, Stanford, CA 94305, USA.