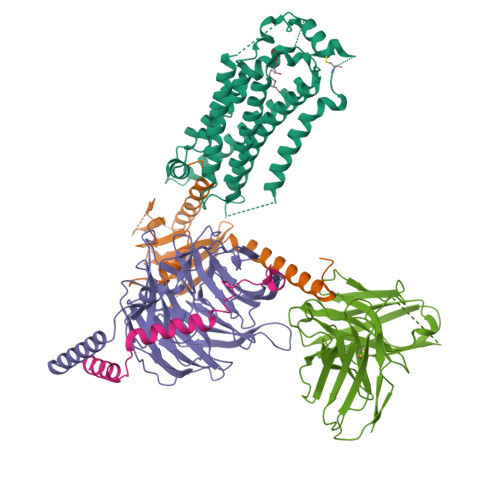
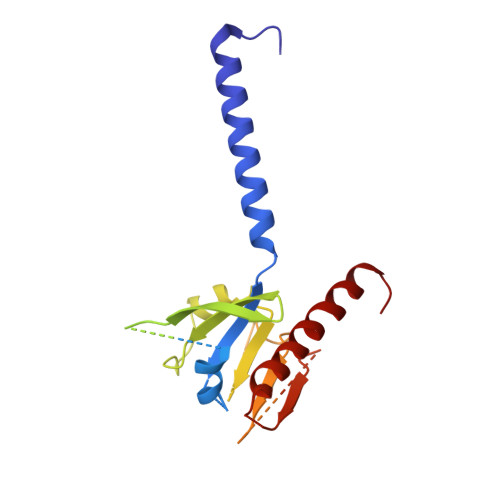
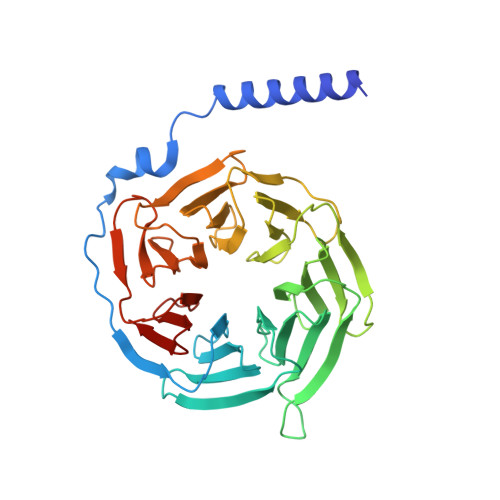
Structural basis of sphingosine-1-phosphate receptor 1 activation and biased agonism.
Xu, Z., Ikuta, T., Kawakami, K., Kise, R., Qian, Y., Xia, R., Sun, M.X., Zhang, A., Guo, C., Cai, X.H., Huang, Z., Inoue, A., He, Y.(2022) Nat Chem Biol 18: 281-288
- PubMed: 34937912
- DOI: https://doi.org/10.1038/s41589-021-00930-3
- Primary Citation of Related Structures:
7EO2, 7EO4, 7WF7 - PubMed Abstract:
Sphingosine-1-phosphate receptor 1 (S1PR1) is a master regulator of lymphocyte egress from the lymph node and an established drug target for multiple sclerosis (MS). Mechanistically, therapeutic S1PR1 modulators activate the receptor yet induce sustained internalization through a potent association with β-arrestin. However, a structural basis of biased agonism remains elusive. Here, we report the cryo-electron microscopy (cryo-EM) structures of G i -bound S1PR1 in complex with S1P, fingolimod-phosphate (FTY720-P) and siponimod (BAF312). In combination with functional assays and molecular dynamics (MD) studies, we reveal that the β-arrestin-biased ligands direct a distinct activation path in S1PR1 through the extensive interplay between the PIF and the NPxxY motifs. Specifically, the intermediate flipping of W269 6.48 and the retained interaction between F265 6.44 and N307 7.49 are the key features of the β-arrestin bias. We further identify ligand-receptor interactions accounting for the S1PR subtype specificity of BAF312. These structural insights provide a rational basis for designing novel signaling-biased S1PR modulators.
Organizational Affiliation:
Laboratory of Receptor Structure and Signaling, The HIT Center for Life Sciences, Harbin Institute of Technology, Harbin, China.