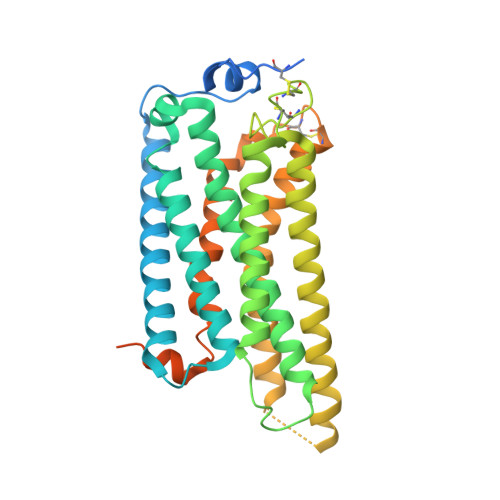
Structure of the active G i -coupled human lysophosphatidic acid receptor 1 complexed with a potent agonist.
Akasaka, H., Tanaka, T., Sano, F.K., Matsuzaki, Y., Shihoya, W., Nureki, O.(2022) Nat Commun 13: 5417-5417
- PubMed: 36109516
- DOI: https://doi.org/10.1038/s41467-022-33121-2
- Primary Citation of Related Structures:
7YU3, 7YU4, 7YU5, 7YU6, 7YU7, 7YU8 - PubMed Abstract:
Lysophosphatidic acid receptor 1 (LPA 1 ) is one of the six G protein-coupled receptors activated by the bioactive lipid, lysophosphatidic acid (LPA). LPA 1 is a drug target for various diseases, including cancer, inflammation, and neuropathic pain. Notably, LPA 1 agonists have potential therapeutic value for obesity and urinary incontinence. Here, we report a cryo-electron microscopy structure of the active human LPA 1 -G i complex bound to ONO-0740556, an LPA analog with more potent activity against LPA 1 . Our structure elucidated the details of the agonist binding mode and receptor activation mechanism mediated by rearrangements of transmembrane segment 7 and the central hydrophobic core. A structural comparison of LPA 1 and other phylogenetically-related lipid-sensing GPCRs identified the structural determinants for lipid preference of LPA 1 . Moreover, we characterized the structural polymorphisms at the receptor-G-protein interface, which potentially reflect the G-protein dissociation process. Our study provides insights into the detailed mechanism of LPA 1 binding to agonists and paves the way toward the design of drug-like agonists targeting LPA 1 .
Organizational Affiliation:
Department of Biological Sciences, Graduate School of Science, The University of Tokyo, Bunkyo, Tokyo, 113-0033, Japan.