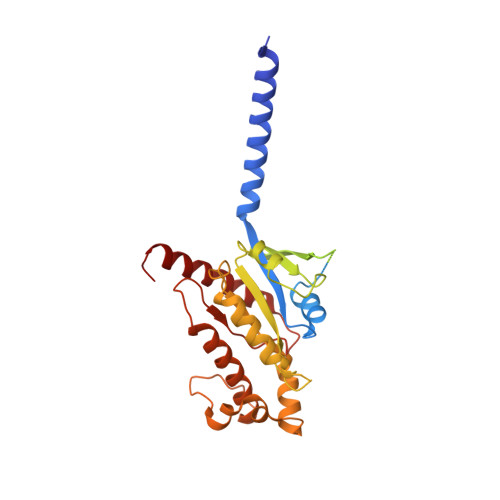
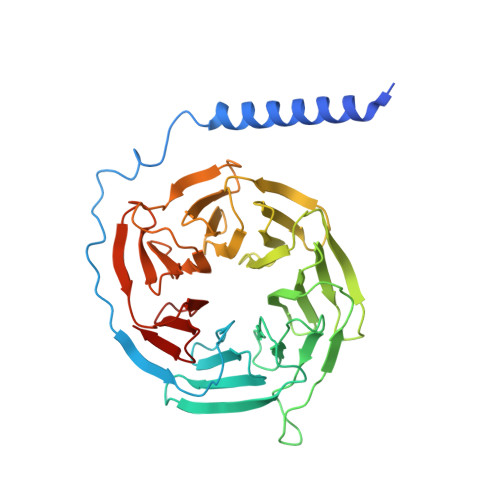
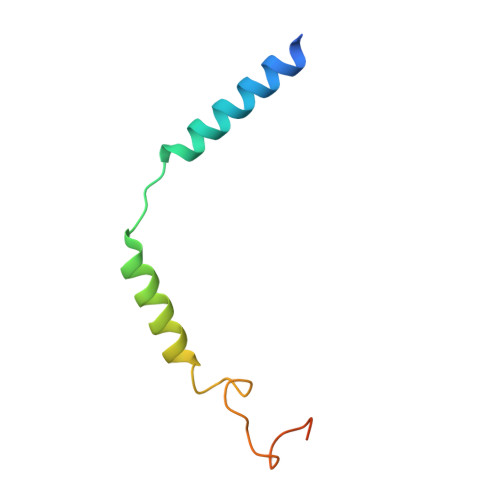
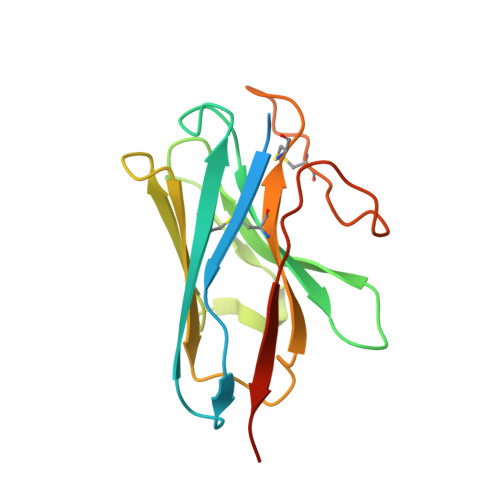
Ligand recognition and allosteric regulation of DRD1-Gs signaling complexes.
Xiao, P., Yan, W., Gou, L., Zhong, Y.N., Kong, L., Wu, C., Wen, X., Yuan, Y., Cao, S., Qu, C., Yang, X., Yang, C.C., Xia, A., Hu, Z., Zhang, Q., He, Y.H., Zhang, D.L., Zhang, C., Hou, G.H., Liu, H., Zhu, L., Fu, P., Yang, S., Rosenbaum, D.M., Sun, J.P., Du, Y., Zhang, L., Yu, X., Shao, Z.(2021) Cell 184: 943-956.e18
- PubMed: 33571432
- DOI: https://doi.org/10.1016/j.cell.2021.01.028
- Primary Citation of Related Structures:
7CKW, 7CKX, 7CKY, 7CKZ, 7CRH - PubMed Abstract:
Dopamine receptors, including D1- and D2-like receptors, are important therapeutic targets in a variety of neurological syndromes, as well as cardiovascular and kidney diseases. Here, we present five cryoelectron microscopy (cryo-EM) structures of the dopamine D1 receptor (DRD1) coupled to Gs heterotrimer in complex with three catechol-based agonists, a non-catechol agonist, and a positive allosteric modulator for endogenous dopamine. These structures revealed that a polar interaction network is essential for catecholamine-like agonist recognition, whereas specific motifs in the extended binding pocket were responsible for discriminating D1- from D2-like receptors. Moreover, allosteric binding at a distinct inner surface pocket improved the activity of DRD1 by stabilizing endogenous dopamine interaction at the orthosteric site. DRD1-Gs interface revealed key features that serve as determinants for G protein coupling. Together, our study provides a structural understanding of the ligand recognition, allosteric regulation, and G protein coupling mechanisms of DRD1.
Organizational Affiliation:
Division of Nephrology and Kidney Research Institute, State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China; Key Laboratory Experimental Teratology of the Ministry of Education and Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, Shandong 250012, China.