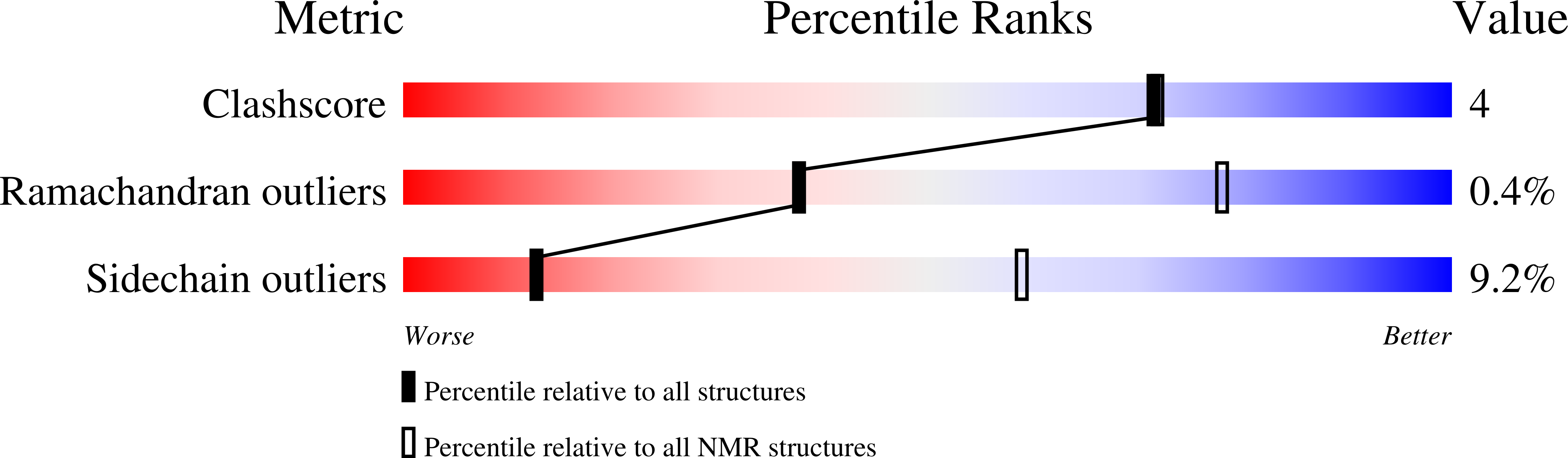An in vitro reconstituted U1 snRNP allows the study of the disordered regions of the particle and the interactions with proteins and ligands.
Campagne, S., de Vries, T., Malard, F., Afanasyev, P., Dorn, G., Dedic, E., Kohlbrecher, J., Boehringer, D., Clery, A., Allain, F.H.(2021) Nucleic Acids Res 49: e63-e63
- PubMed: 33677607
- DOI: https://doi.org/10.1093/nar/gkab135
- Primary Citation of Related Structures:
7AEP - PubMed Abstract:
U1 small nuclear ribonucleoparticle (U1 snRNP) plays a central role during RNA processing. Previous structures of U1 snRNP revealed how the ribonucleoparticle is organized and recognizes the pre-mRNA substrate at the exon-intron junction. As with many other ribonucleoparticles involved in RNA metabolism, U1 snRNP contains extensions made of low complexity sequences. Here, we developed a protocol to reconstitute U1 snRNP in vitro using mostly full-length components in order to perform liquid-state NMR spectroscopy. The accuracy of the reconstitution was validated by probing the shape and structure of the particle by SANS and cryo-EM. Using an NMR spectroscopy-based approach, we probed, for the first time, the U1 snRNP tails at atomic detail and our results confirm their high degree of flexibility. We also monitored the labile interaction between the splicing factor PTBP1 and U1 snRNP and validated the U1 snRNA stem loop 4 as a binding site for the splicing regulator on the ribonucleoparticle. Altogether, we developed a method to probe the intrinsically disordered regions of U1 snRNP and map the interactions controlling splicing regulation. This approach could be used to get insights into the molecular mechanisms of alternative splicing and screen for potential RNA therapeutics.
Organizational Affiliation:
Institute of Biochemistry, Department of Biology, ETH Zurich, Hönggerbergring 64, CH-8093 Zürich, Switzerland.