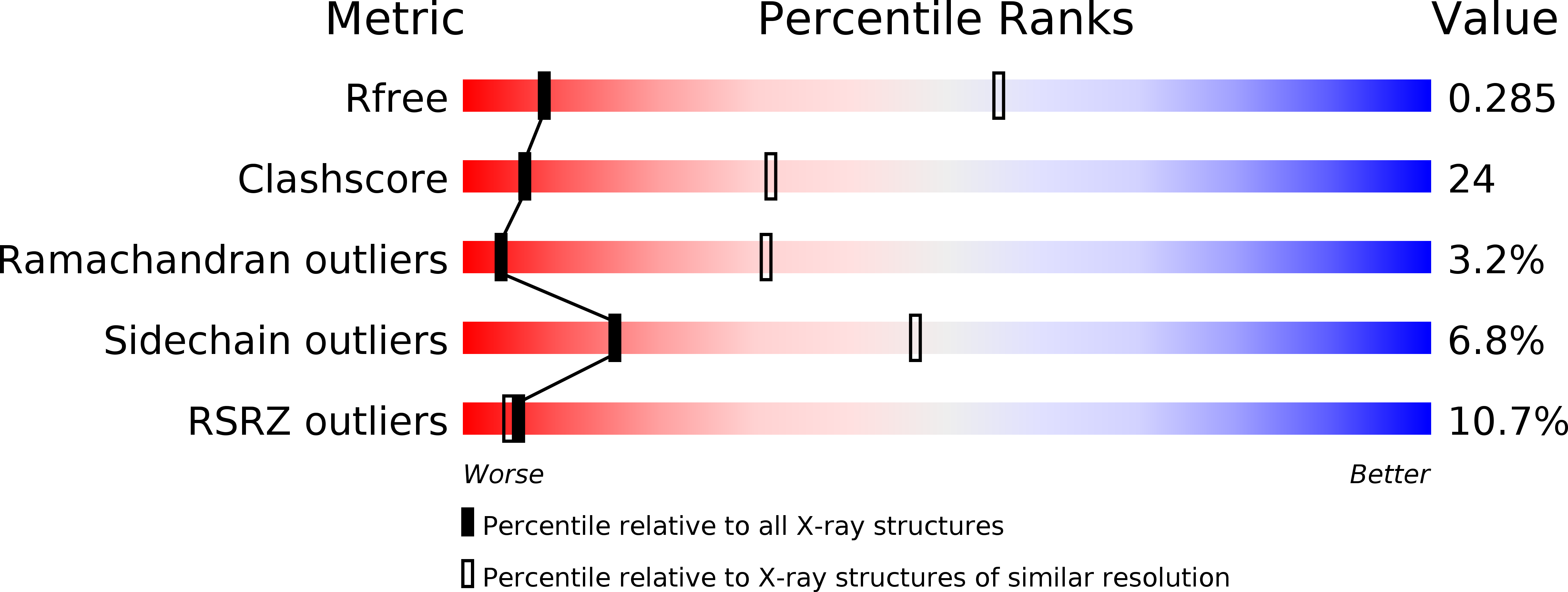
Controlling Photosynthetic Excitons by Selective Pigment Photooxidation.
Leiger, K., Linnanto, J.M., Ratsep, M., Timpmann, K., Ashikhmin, A.A., Moskalenko, A.A., Fufina, T.Y., Gabdulkhakov, A.G., Freiberg, A.(2019) J Phys Chem B 123: 29-38
- PubMed: 30543422
- DOI: https://doi.org/10.1021/acs.jpcb.8b08083
- Primary Citation of Related Structures:
6Q53 - PubMed Abstract:
As a basis of photosynthesis, photoinduced oxidation of (bacterio)chlorophyll molecules in the special reaction center complexes has been a subject of extensive research. In contrast, the generally harmful photooxidation of antenna chromoproteins has received much less attention. Here, we have established the permanent structural changes in the LH2 antenna bacteriochlorophyll-protein complex from a sulfur photosynthetic purple bacterium Ectothiorhodospira haloalkaliphila taking place at physiological conditions upon intense optical irradiation. To this end, a crystal structure of the LH2 complex from E. haloalkaliphila was first resolved by X-ray diffraction to 3.7 Å, verifying a great similarity with the earlier structure from Phaesporillum molischianum. Analysis of the various steady-state and picosecond time-resolved optical spectroscopy data and related model simulations then confirmed that the major spectral effects observed-bleaching and blue-shifting of the B850 exciton band and correlated emergence of a higher-energy C700 exciton band-are associated with photooxidation of increasing numbers of B850 bacteriochlorophylls into 3-acetyl-chlorophylls, with no noticeable damage to the pigment-binding protein scaffold. A prospective noninvasive method for an in situ optical control of excitons by selective photooxidation of pigment chromophores was thus revealed and demonstrated in a structurally well-defined native system.
Organizational Affiliation:
Institute of Physics , University of Tartu , W. Ostwaldi 1 , Tartu 50411 , Estonia.