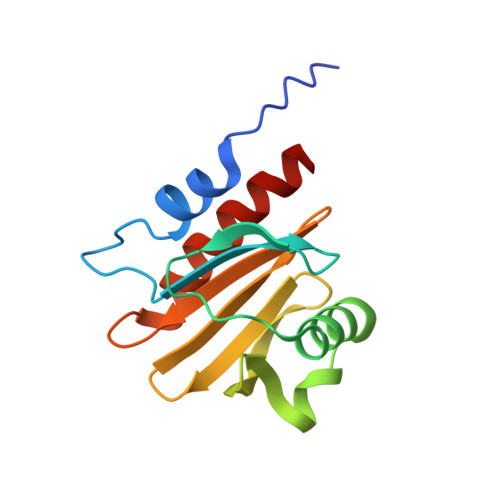
Structural and computational examination of theArabidopsisprofilin-Poly-P complex reveals mechanistic details in profilin-regulated actin assembly.
Qiao, Z., Sun, H., Ng, J.T.Y., Ma, Q., Koh, S.H., Mu, Y., Miao, Y., Gao, Y.G.(2019) J Biol Chem 294: 18650-18661
- PubMed: 31653702
- DOI: https://doi.org/10.1074/jbc.RA119.011307
- Primary Citation of Related Structures:
6IQF, 6IQI, 6IQJ, 6IQK - PubMed Abstract:
Profilins are abundant cytosolic proteins that are universally expressed in eukaryotes and that regulate actin filament elongation by binding to both monomeric actin (G-actin) and formin proteins. The atypical profilin Arabidopsis AtPRF3 has been reported to cooperate with canonical profilin isoforms in suppressing formin-mediated actin polymerization during plant innate immunity responses. AtPRF3 has a 37-amino acid-long N-terminal extension (NTE), and its suppressive effect on actin assembly is derived from enhanced interaction with the polyproline (Poly-P) of the formin AtFH1. However, the molecular mechanism remains unclear. Here, we solved the crystal structures of AtPRF3Δ22 and AtPRF3Δ37, as well as AtPRF2 apo form and in complex with AtFH1 Poly-P at 1.5-3.6 Å resolutions. By combining these structures with molecular modeling, we found that AtPRF3Δ22 NTE has high plasticity, with a primary "closed" conformation that can adopt an open conformation that enables Poly-P binding. Furthermore, using molecular dynamics simulation and free-energy calculations of protein-protein binding, along with experimental validation, we show that the AtPRF3Δ22 binds to Poly-P in an adaptive manner, thereby enabling different binding modes that maintain the interaction through disordered sequences. Together, our structural and simulation results suggest that the dynamic conformational changes of the AtPRF3 NTE upon Poly-P binding modulate their interactions to fine-tune formin-mediated actin assembly.
Organizational Affiliation:
School of Biological Sciences, Nanyang Technological University, 637551 Singapore.