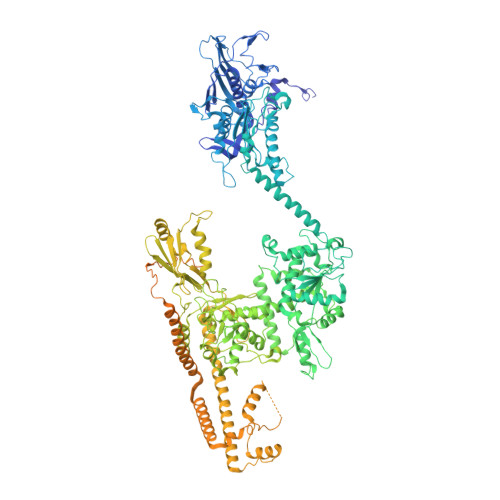
Structural basis for allosteric regulation of Human Topoisomerase II alpha.
Vanden Broeck, A., Lotz, C., Drillien, R., Haas, L., Bedez, C., Lamour, V.(2021) Nat Commun 12: 2962-2962
- PubMed: 34016969
- DOI: https://doi.org/10.1038/s41467-021-23136-6
- Primary Citation of Related Structures:
6ZY5, 6ZY6, 6ZY7, 6ZY8 - PubMed Abstract:
The human type IIA topoisomerases (Top2) are essential enzymes that regulate DNA topology and chromosome organization. The Topo IIα isoform is a prime target for antineoplastic compounds used in cancer therapy that form ternary cleavage complexes with the DNA. Despite extensive studies, structural information on this large dimeric assembly is limited to the catalytic domains, hindering the exploration of allosteric mechanism governing the enzyme activities and the contribution of its non-conserved C-terminal domain (CTD). Herein we present cryo-EM structures of the entire human Topo IIα nucleoprotein complex in different conformations solved at subnanometer resolutions (3.6-7.4 Å). Our data unveils the molecular determinants that fine tune the allosteric connections between the ATPase domain and the DNA binding/cleavage domain. Strikingly, the reconstruction of the DNA-binding/cleavage domain uncovers a linker leading to the CTD, which plays a critical role in modulating the enzyme's activities and opens perspective for the analysis of post-translational modifications.
Organizational Affiliation:
Université de Strasbourg, CNRS, INSERM, Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC), Illkirch, France.