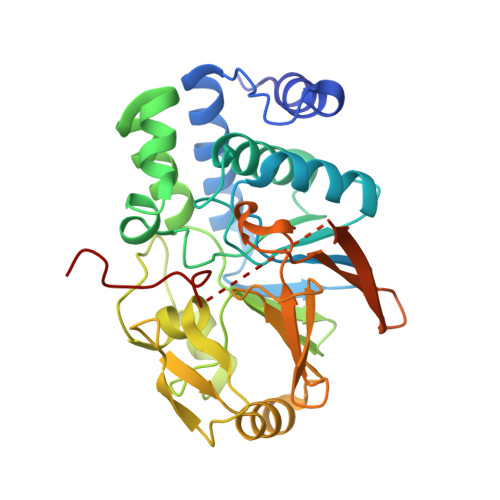
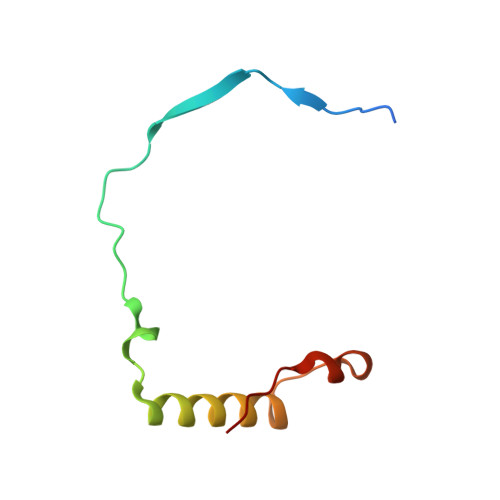
Molecular basis for substrate specificity of the Phactr1/PP1 phosphatase holoenzyme.
Fedoryshchak, R.O., Prechova, M., Butler, A., Lee, R., O'Reilly, N., Flynn, H.R., Snijders, A.P., Eder, N., Ultanir, S., Mouilleron, S., Treisman, R.(2020) Elife 9
- PubMed: 32975518
- DOI: https://doi.org/10.7554/eLife.61509
- Primary Citation of Related Structures:
6ZEE, 6ZEF, 6ZEG, 6ZEH, 6ZEI, 6ZEJ - PubMed Abstract:
PPP-family phosphatases such as PP1 have little intrinsic specificity. Cofactors can target PP1 to substrates or subcellular locations, but it remains unclear how they might confer sequence-specificity on PP1. The cytoskeletal regulator Phactr1 is a neuronally enriched PP1 cofactor that is controlled by G-actin. Structural analysis showed that Phactr1 binding remodels PP1's hydrophobic groove, creating a new composite surface adjacent to the catalytic site. Using phosphoproteomics, we identified mouse fibroblast and neuronal Phactr1/PP1 substrates, which include cytoskeletal components and regulators. We determined high-resolution structures of Phactr1/PP1 bound to the dephosphorylated forms of its substrates IRSp53 and spectrin αII. Inversion of the phosphate in these holoenzyme-product complexes supports the proposed PPP-family catalytic mechanism. Substrate sequences C-terminal to the dephosphorylation site make intimate contacts with the composite Phactr1/PP1 surface, which are required for efficient dephosphorylation. Sequence specificity explains why Phactr1/PP1 exhibits orders-of-magnitude enhanced reactivity towards its substrates, compared to apo-PP1 or other PP1 holoenzymes.
Organizational Affiliation:
Signalling and Transcription Laboratory, The Francis Crick Institute, London, United Kingdom.