Anticancer immunity induced by a synthetic tumor-targeted CD137 agonist.
Upadhyaya, P., Lahdenranta, J., Hurov, K., Battula, S., Dods, R., Haines, E., Kleyman, M., Kristensson, J., Kublin, J., Lani, R., Ma, J., Mudd, G., Repash, E., Van Rietschoten, K., Stephen, T., You, F., Harrison, H., Chen, L., McDonnell, K., Brandish, P., Keen, N.(2021) J Immunother Cancer 9
- PubMed: 33500260
- DOI: https://doi.org/10.1136/jitc-2020-001762
- Primary Citation of Related Structures:
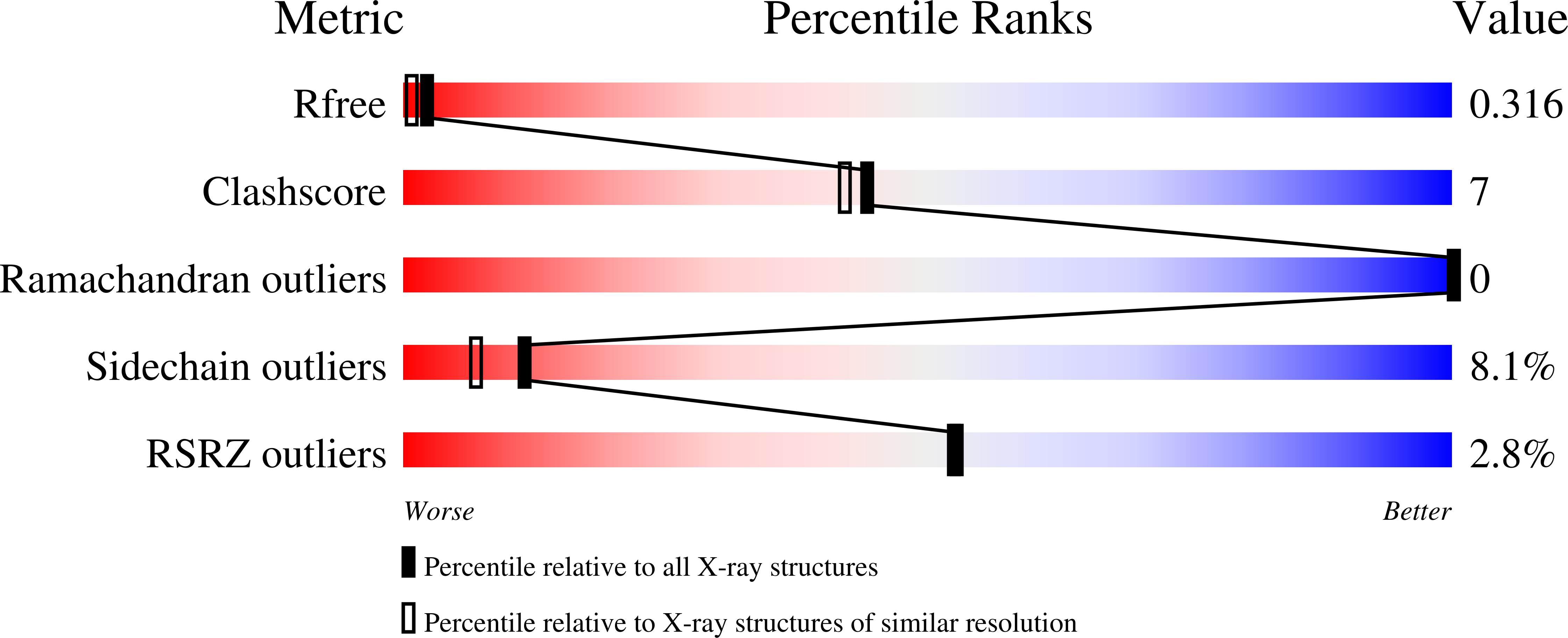
6Y8K - PubMed Abstract:
In contrast to immune checkpoint inhibitors, the use of antibodies as agonists of immune costimulatory receptors as cancer therapeutics has largely failed. We sought to address this problem using a new class of modular synthetic drugs, termed tumor-targeted immune cell agonists (TICAs), based on constrained bicyclic peptides ( Bicycles ). Phage libraries displaying Bicycles were panned for binders against tumor necrosis factor (TNF) superfamily receptors CD137 and OX40, and tumor antigens EphA2, Nectin-4 and programmed death ligand 1. The CD137 and OX40 Bicycles were chemically conjugated to tumor antigen Bicycles with different linkers and stoichiometric ratios of binders to obtain a library of low molecular weight TICAs (MW <8 kDa). The TICAs were evaluated in a suite of in vitro and in vivo assays to characterize their pharmacology and mechanism of action. Linking Bicycles against costimulatory receptors (e.g., CD137) to Bicycles against tumor antigens (e.g., EphA2) created potent agonists that activated the receptors selectively in the presence of tumor cells expressing these antigens. An EphA2/CD137 TICA (BCY12491) efficiently costimulated human peripheral blood mononuclear cells in vitro in the presence of EphA2 expressing tumor cell lines as measured by the increased secretion of interferon γ and interleukin-2. Treatment of C57/Bl6 mice transgenic for the human CD137 extracellular domain (huCD137) bearing EphA2-expressing MC38 tumors with BCY12491 resulted in the infiltration of CD8+ T cells, elimination of tumors and generation of immunological memory. BCY12491 was cleared quickly from the circulation (plasma t 1/2 in mice of 1-2 hr), yet intermittent dosing proved effective. Tumor target-dependent CD137 agonism using a novel chemical approach (TICAs) afforded elimination of tumors with only intermittent dosing suggesting potential for a wide therapeutic index in humans. This work unlocks a new path to effective cancer immunotherapy via agonism of TNF superfamily receptors.
Organizational Affiliation:
Bicycle Therapeutics, Lexington, Massachusetts, USA.