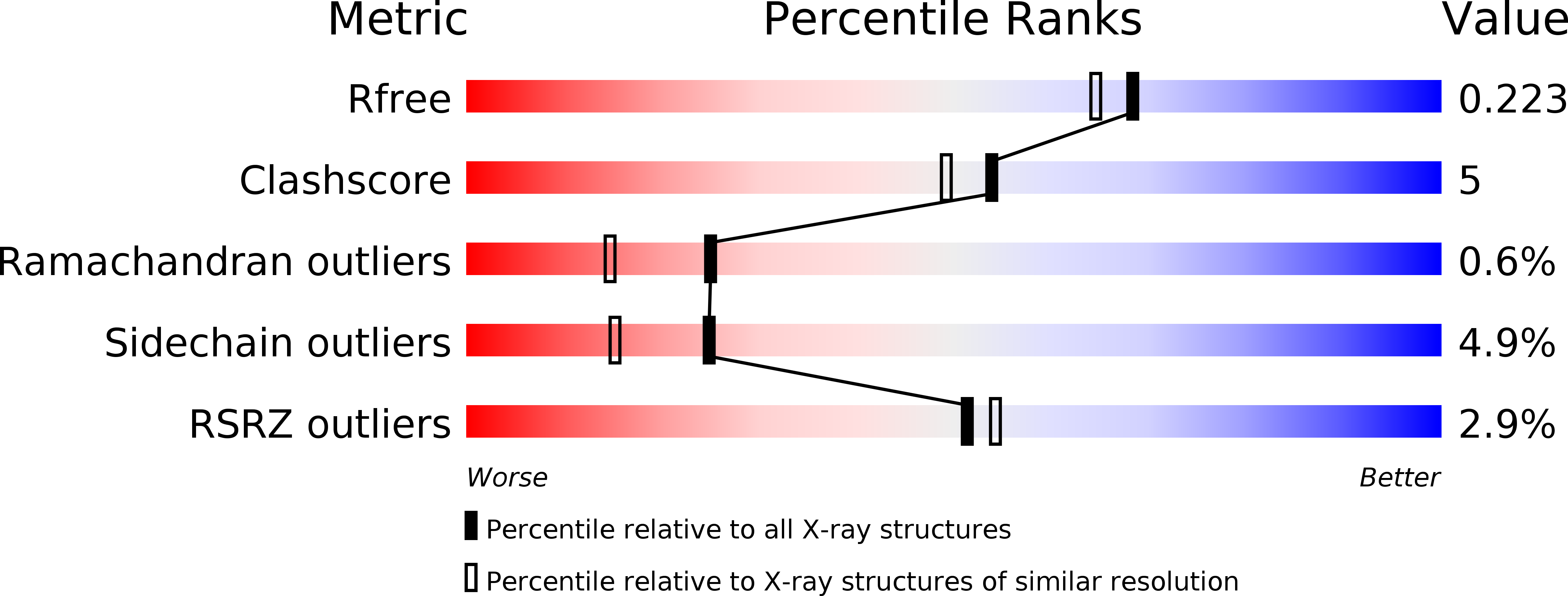
TF3
Query on TF3
Download Ideal Coordinates CCD File
| B [auth AAA] | N-(2-AMINOETHYL)-2-{3-CHLORO-4-[(4-ISOPROPYLBENZYL)OXY]PHENYL} ACETAMIDE
C20 H25 Cl N2 O2
DFXJYVQAAFOZDP-UHFFFAOYSA-N |  | |
NLZ (Subject of Investigation/LOI)
Query on NLZ
Download Ideal Coordinates CCD File
| C [auth AAA] | 2,4-bis(oxidanyl)benzamide
C7 H7 N O3
IIUJCQYKTGNRHH-UHFFFAOYSA-N |  | |
DMS
Query on DMS
Download Ideal Coordinates CCD File
| D [auth AAA] | DIMETHYL SULFOXIDE
C2 H6 O S
IAZDPXIOMUYVGZ-UHFFFAOYSA-N |  | |
CL
Query on CL
Download Ideal Coordinates CCD File
| E [auth AAA] | CHLORIDE ION
Cl
VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | |