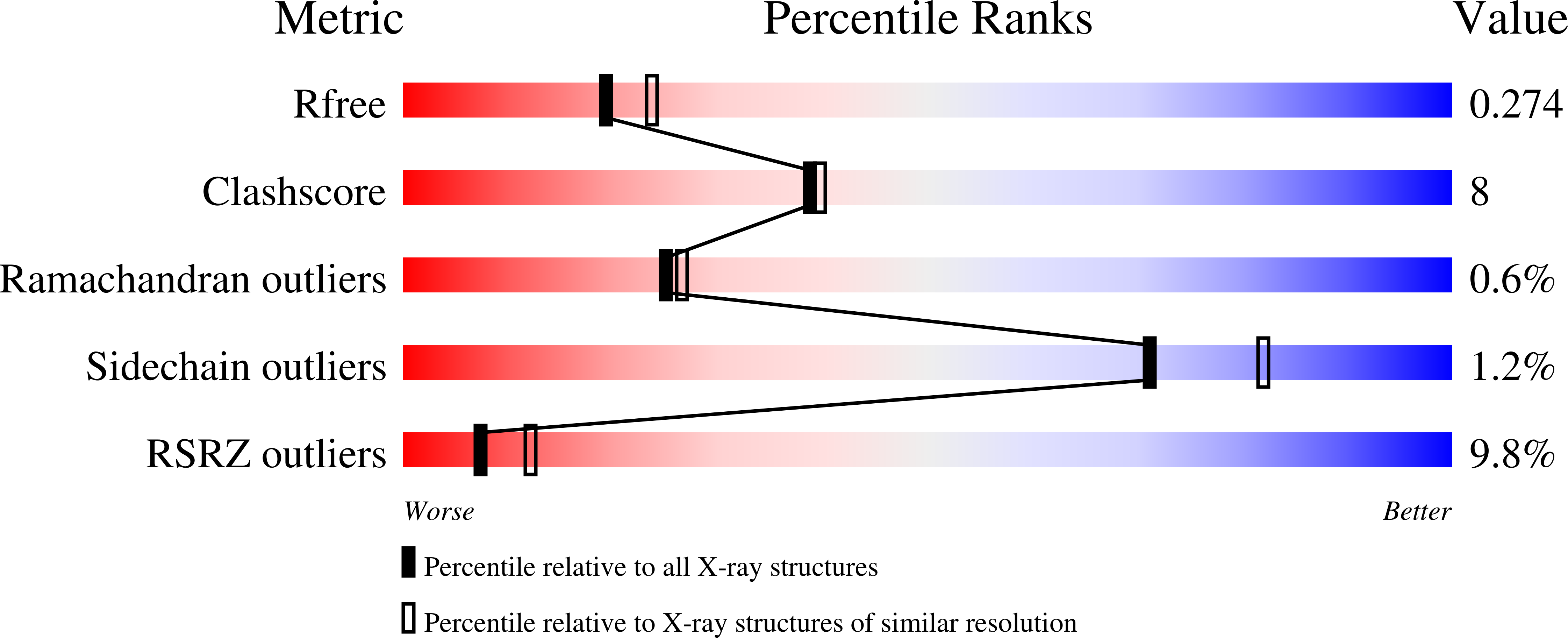
Design, synthesis and evaluation of potent and selective inhibitors of mono-(ADP-ribosyl)transferases PARP10 and PARP14.
Holechek, J., Lease, R., Thorsell, A.G., Karlberg, T., McCadden, C., Grant, R., Keen, A., Callahan, E., Schuler, H., Ferraris, D.(2018) Bioorg Med Chem Lett 28: 2050-2054
- PubMed: 29748053
- DOI: https://doi.org/10.1016/j.bmcl.2018.04.056
- Primary Citation of Related Structures:
6G0W - PubMed Abstract:
A series of diaryl ethers were designed and synthesized to discern the structure activity relationships against the two closely related mono-(ADP-ribosyl)transferases PARP10 and PARP14. Structure activity studies identified 8b as a sub-micromolar inhibitor of PARP10 with ∼15-fold selectivity over PARP14. In addition, 8k and 8m were discovered to have sub-micromolar potency against PARP14 and demonstrated moderate selectivity over PARP10. A crystal structure of the complex of PARP14 and 8b shows binding of the compound in a novel hydrophobic pocket and explains both potency and selectivity over other PARP family members. In addition, 8b, 8k and 8m also demonstrate selectivity over PARP1. Together, this study identified novel, potent and metabolically stable derivatives to use as chemical probes for these biologically interesting therapeutic targets.
Organizational Affiliation:
McDaniel College, 2 College Hill, Westminster, MD 21157, United States.