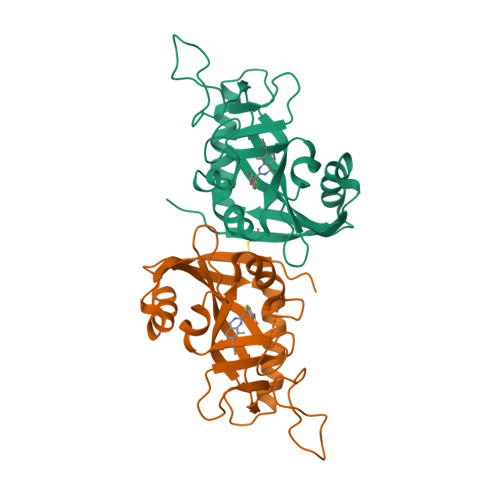
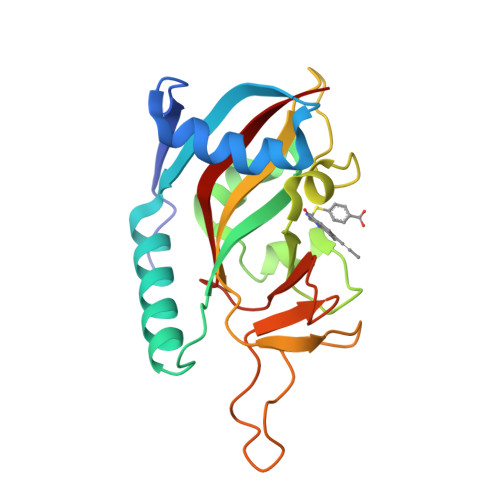
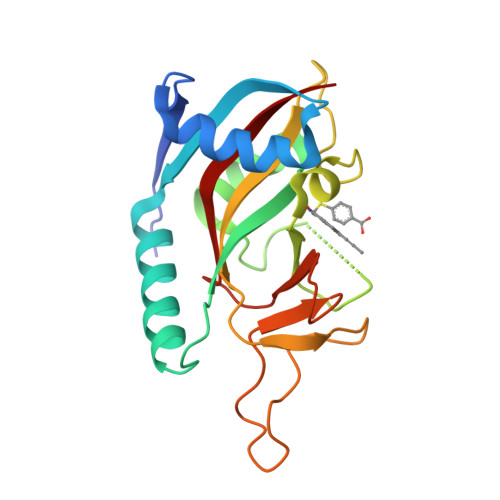
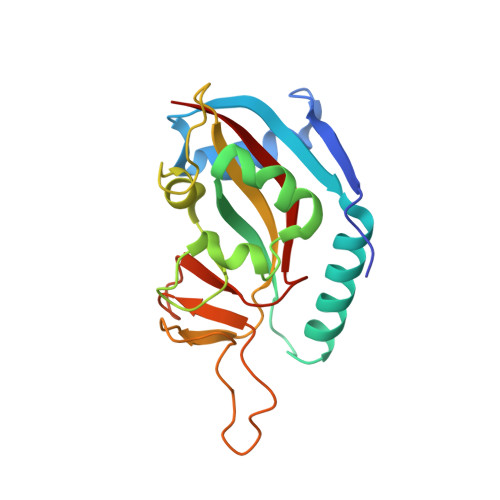
A Potent and Selective PARP11 Inhibitor Suggests Coupling between Cellular Localization and Catalytic Activity.
Kirby, I.T., Kojic, A., Arnold, M.R., Thorsell, A.G., Karlberg, T., Vermehren-Schmaedick, A., Sreenivasan, R., Schultz, C., Schuler, H., Cohen, M.S.(2018) Cell Chem Biol 25: 1547-1553.e12
- PubMed: 30344052
- DOI: https://doi.org/10.1016/j.chembiol.2018.09.011
- Primary Citation of Related Structures:
6FYM, 6FZM - PubMed Abstract:
Poly-ADP-ribose polymerases (PARPs1-16) play pivotal roles in diverse cellular processes. PARPs that catalyze poly-ADP-ribosylation (PARylation) are the best characterized PARP family members because of the availability of potent and selective inhibitors for these PARPs. There has been comparatively little success in developing selective small-molecule inhibitors of PARPs that catalyze mono-ADP-ribosylation (MARylation), limiting our understanding of the cellular role of MARylation. Here we describe the structure-guided design of inhibitors of PARPs that catalyze MARylation. The most selective analog, ITK7, potently inhibits the MARylation activity of PARP11, a nuclear envelope-localized PARP. ITK7 is greater than 200-fold selective over other PARP family members. Using live-cell imaging, we show that ITK7 causes PARP11 to dissociate from the nuclear envelope. These results suggest that the cellular localization of PARP11 is regulated by its catalytic activity.
Organizational Affiliation:
Program in Chemical Biology, Oregon Health & Science University, Portland, OR 97210, USA; Department of Physiology and Pharmacology, Oregon Health & Science University, Portland, OR 97210, United States.