Design, Synthesis, and Biological Evaluation of Retinoic Acid-Related Orphan Receptor gamma t (ROR gamma t) Agonist Structure-Based Functionality Switching Approach from In House ROR gamma t Inverse Agonist to ROR gamma t Agonist.
Yukawa, T., Nara, Y., Kono, M., Sato, A., Oda, T., Takagi, T., Sato, T., Banno, Y., Taya, N., Imada, T., Shiokawa, Z., Negoro, N., Kawamoto, T., Koyama, R., Uchiyama, N., Skene, R., Hoffman, I., Chen, C.H., Sang, B., Snell, G., Katsuyama, R., Yamamoto, S., Shirai, J.(2019) J Med Chem 62: 1167-1179
- PubMed: 30652849
- DOI: https://doi.org/10.1021/acs.jmedchem.8b01181
- Primary Citation of Related Structures:
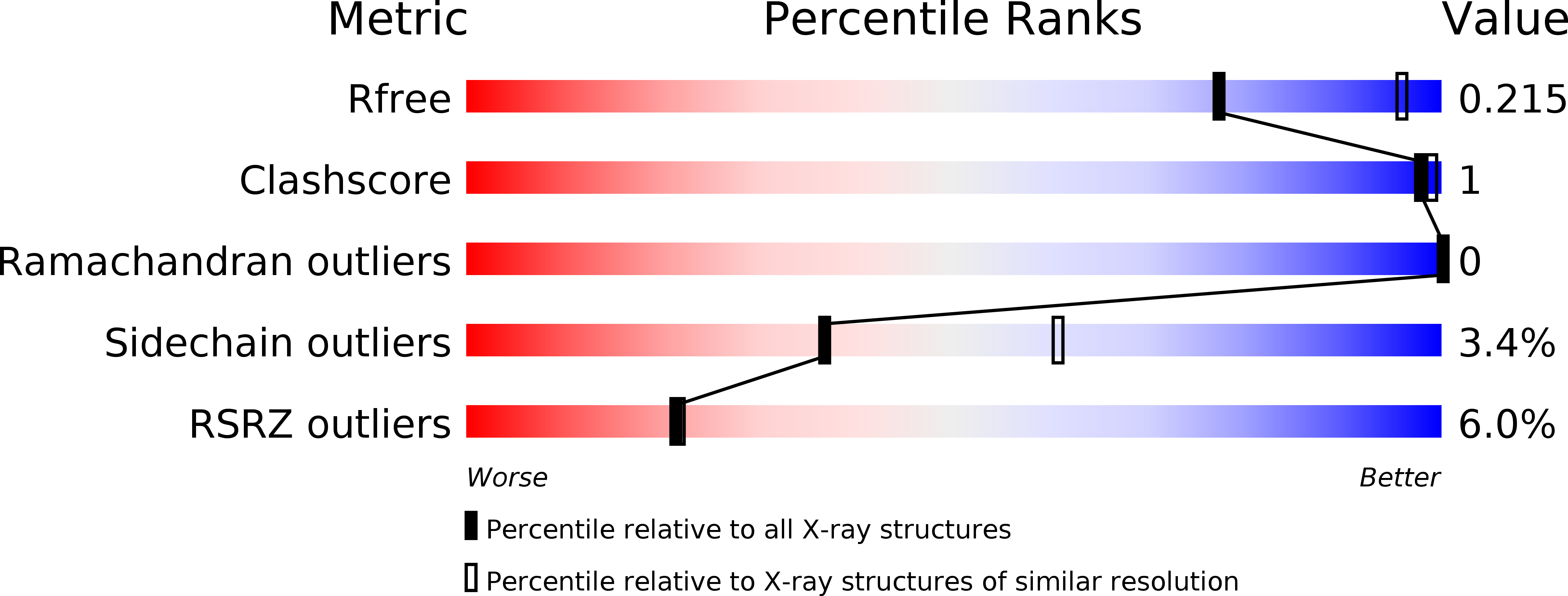
6E3E, 6E3G - PubMed Abstract:
Retinoic acid receptor-related orphan receptor γt (RORγt) agonists are expected to provide a novel class of immune-activating anticancer drugs via activation of Th17 cells and Tc17 cells. Herein, we describe a novel structure-based functionality switching approach from in house well-optimized RORγt inverse agonists to potent RORγt agonists. We succeeded in the identification of potent RORγt agonist 5 without major chemical structure change. The biochemical response was validated by molecular dynamics simulation studies that showed a helix 12 stabilization effect of RORγt agonists. These results indicate that targeting helix 12 is an attractive and novel medicinal chemistry strategy for switching existing RORγt inverse agonists to agonists.
Organizational Affiliation:
Pharmaceutical Research Division , Takeda Pharmaceutical Company Limited , 26-1 Muraoka-Higashi 2-chome , Fujisawa , Kanagawa 251-8555 , Japan.